Bumps on the Road: The Way to Clean Relaxation Dispersion Magic-Angle Spinning NMR
- PMID: 40748291
- PMCID: PMC12356580
- DOI: 10.1021/jacs.5c09057
Bumps on the Road: The Way to Clean Relaxation Dispersion Magic-Angle Spinning NMR
Abstract
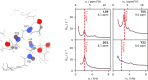
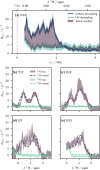
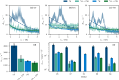
Microsecond-to-millisecond motions are instrumental for many biomolecular functions, including enzymatic activity and ligand binding. Bloch-McConnell Relaxation Dispersion (BMRD) Nuclear Magnetic Resonance (NMR) spectroscopy is a key technique for studying these dynamic processes. While BMRD experiments are routinely used to probe protein motions in solution, the experiment is more demanding in the solid state, where dipolar couplings complicate the spin dynamics. It is believed that high deuteration levels are required and sufficient to obtain accurate and quantitative data. Here we show that even under fast magic-angle spinning and high levels of deuteration artifactual "bumps" in 15N R1ρ BMRD profiles are common. The origin of these artifacts is identified as a second-order three-spin Mixed Rotational and Rotary Resonance (MIRROR) recoupling condition. These artifacts are found to be a significant confounding factor for the accurate quantification of microsecond protein dynamics using BMRD in the solid state. We show that the application of low-power continuous wave (CW) decoupling simultaneously with the 15N spin-lock leads to the suppression of these conditions and enables quantitative measurements of microsecond exchange in the solid state. Remarkably, the application of decoupling allows the measurement of accurate BMRD even in fully protonated proteins at 100 kHz MAS, thus extending the scope of μs dynamics measurements in MAS NMR.
Figures




Similar articles
-
Unbiased clustering of residues undergoing synchronous motions in proteins using NMR spin relaxation data.Biophys Chem. 2025 May-Jun;320-321:107411. doi: 10.1016/j.bpc.2025.107411. Epub 2025 Feb 15. Biophys Chem. 2025. PMID: 39983456
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Probing rare and short-lived conformational states in nucleic acids using off-resonance carbonyl and guanidino carbon R1ρ relaxation dispersion.J Magn Reson. 2025 Aug;377:107910. doi: 10.1016/j.jmr.2025.107910. Epub 2025 May 29. J Magn Reson. 2025. PMID: 40466235
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Chemical Profiling and Quality Assessment of Food Products Employing Magnetic Resonance Technologies.Foods. 2025 Jul 9;14(14):2417. doi: 10.3390/foods14142417. Foods. 2025. PMID: 40724238 Free PMC article. Review.
References
-
- Joshi S. Y., S A. D.. A review of advancements in coarse-grained molecular dynamics simulations. Mol. Simul. 2021;47:786–803. doi: 10.1080/08927022.2020.1828583. - DOI
-
- Martin-Garcia J. M.. Protein Dynamics and Time Resolved Protein Crystallography at Synchrotron Radiation Sources: Past, Present and Future. Crystals. 2021;11:521. doi: 10.3390/cryst11050521. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

