Genomic and secretomic analyses of Blastobotrys yeasts reveal key xylanases for biomass decomposition
- PMID: 40748385
- PMCID: PMC12316802
- DOI: 10.1007/s00253-025-13556-5
Genomic and secretomic analyses of Blastobotrys yeasts reveal key xylanases for biomass decomposition
Abstract
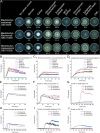
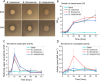
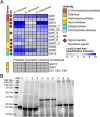
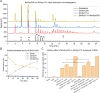
Xylanolytic enzyme systems in ascomycetous yeasts remain underexplored, despite the presence of yeasts in various xylan-rich ecological niches. In this study, we investigated the secreted xylanolytic machineries of three Blastobotrys species-B. mokoenaii, B. illinoisensis, and B. malaysiensis-by integrating genome annotation, bioinformatics, and secretome analyses of cultures grown on beechwood glucuronoxylan. Our findings demonstrate that these yeasts effectively hydrolyze xylan through the secretion of xylanases from the glycoside hydrolase (GH) family 11, which play a central role in cleaving the xylan backbone. Additionally, the yeasts produce a diverse array of other CAZymes, including members of GH families 3, 5, and 67, with putative roles in xylan degradation. We also report on the heterologous expression and functional characterization of the GH30_7 xylanase BmXyn30A from B. mokoenaii, which exhibits both glucuronoxylanase and xylobiohydrolase activities. We demonstrate additive effects between GH family 30 BmXyn30A and GH family 11 BmXyn11A during the hydrolysis of beechwood glucuronoxylan, where the enzymes exhibit complementary roles that enhance the deconstruction of this complex hemicellulose substrate. These findings broaden our understanding of the xylanolytic systems in yeasts and underscore the potential of Blastobotrys species as cell factories and natural xylanase producers. The enzymes they produce hold promise for biorefining applications, enabling efficient utilization of renewable xylan-rich plant biomass resources. KEY POINTS: • Extracellular GH11 xylanases dominate glucuronoxylan degradation in Blastobotrys yeasts. • Yeast GH30_7 enzyme shows multifaceted activity, supporting complex xylan breakdown. • Blastobotrys yeasts show promise as cell factories for industrial biotechnology applications.
Keywords: GH11; GH30; Glycoside hydrolase; Wood; Xylan; Xylanolytic yeast.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Not applicable. Competing Interests: The authors declare no competing interests.
Figures
Similar articles
-
Yeasts Have Evolved Divergent Enzyme Strategies To Deconstruct and Metabolize Xylan.Microbiol Spectr. 2023 Jun 15;11(3):e0024523. doi: 10.1128/spectrum.00245-23. Epub 2023 Apr 26. Microbiol Spectr. 2023. PMID: 37098941 Free PMC article.
-
CAZyme prediction in ascomycetous yeast genomes guides discovery of novel xylanolytic species with diverse capacities for hemicellulose hydrolysis.Biotechnol Biofuels. 2021 Jul 2;14(1):150. doi: 10.1186/s13068-021-01995-x. Biotechnol Biofuels. 2021. PMID: 34215291 Free PMC article.
-
Identifying promoters to enhance heterologous gene expression in recombinant Saccharomyces cerevisiae strains cultivated on non-native substrates.Appl Microbiol Biotechnol. 2025 Jul 26;109(1):173. doi: 10.1007/s00253-025-13563-6. Appl Microbiol Biotechnol. 2025. PMID: 40715790 Free PMC article.
-
Xylanases of glycoside hydrolase family 30 - An overview.Biotechnol Adv. 2021 Mar-Apr;47:107704. doi: 10.1016/j.biotechadv.2021.107704. Epub 2021 Feb 3. Biotechnol Adv. 2021. PMID: 33548454 Review.
-
Insights into the mechanism of enzymatic hydrolysis of xylan.Appl Microbiol Biotechnol. 2016 Jun;100(12):5205-14. doi: 10.1007/s00253-016-7555-z. Epub 2016 Apr 25. Appl Microbiol Biotechnol. 2016. PMID: 27112349 Review.
References
-
- Boekhout T, Amend AS, El Baidouri F, Gabaldón T, Geml J, Mittelbach M, Robert V, Tan CS, Turchetti B, Vu D, Wang QM, Yurkov A (2022) Trends in yeast diversity discovery. Fungal Divers 114:491–537. 10.1007/s13225-021-00494-6
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

