Myocardial sympathetic distal axon loss in subjects with Lewy pathology in three autopsy cohorts
- PMID: 40748411
- PMCID: PMC12316836
- DOI: 10.1007/s00401-025-02918-y
Myocardial sympathetic distal axon loss in subjects with Lewy pathology in three autopsy cohorts
Abstract
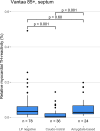
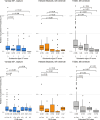
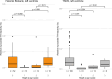
Cardiac manifestations are associated with Lewy body disease, but studies addressing the underlying histopathological mechanisms at the myocardial level are sparse. Here, we generated an artificial intelligence-based algorithm to quantify tyrosine hydroxylase (TH)-immunoreactive sympathetic distal axons at the myocardial level. This novel tool was applied to septal samples of the Vantaa 85 + study (n = 138), which is a population-based autopsy study representing all subjects aged 85 years or older living in the city of Vantaa (southern Finland) in 1991. In addition, the tool was applied to left ventricle samples of the Helsinki Biobank (n = 87) and the forensic Tampere Sudden Death Study (TSDS, n = 127). The level of myocardial TH reactivity was compared between subjects with and without Lewy pathology in the central nervous system in all datasets. In the Vantaa 85 + study, TH reactivity was also compared between subjects with caudo-rostral and amygdala-based subtypes, and potential confounding factors (age at death, sex, myocardial infarction, senile systemic amyloidosis, and diabetes medication) were controlled for using multiple linear regression models. Presence of Lewy pathology was strongly associated with loss of TH reactivity at the myocardial level in all three autopsy cohorts (Vantaa 85 + p = 0.001, Helsinki Biobank p < 0.001, TSDS p < 0.001)). In the Vantaa 85 + study, the caudo-rostral subtype (p < 0.001), but not the amygdala-based subtype (p = 0.60), was associated with myocardial denervation/dysfunction, and this association was independent of other known causes of sympathetic denervation/dysfunction. Caudo-rostral subtype and myocardial infarction were the strongest predictors of myocardial sympathetic denervation/dysfunction in the oldest-old population (Vantaa 85 +). In conclusion, our results show that Lewy pathology in the central nervous system, and particularly its caudo-rostral subtype, is strongly associated with loss of sympathetic distal axons at the myocardial level. We also provide evidence that the caudo-rostral subtype is one of the strongest predictors of myocardial sympathetic denervation/dysfunction in the oldest-old population.
Keywords: Image recognition tool; Lewy body disease; Myocardium; Neurodegeneration; Neuropathology; Post-mortem.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: P.J.T. holds a patent on C9orf72 in the diagnostics and treatment of ALS/FTD and has made paid consultations for Roche, Biogen, Merck, Lundbeck, Sanofi-Genzyme and Novartis unrelated to the subject of this study. H.P. has provided paid pathology consulting services for Aiforia Technologies Oyj in unrelated projects. All other authors report no competing interests. Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Consent to participate: In the Vantaa 85 + study, each participant and/or their relatives have given informed consent for the study and relatives have given written consent for autopsy. In accordance with the Finnish national ethical standards and the Act on the Medical Use of Human Organs, Tissues and Cells (101/2001), which allow using post-mortem samples collected as part of medical or forensic autopsies in research, informed consent was not obtained (or required) for the Helsinki Biobank and TSDS cohorts.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

