Patient satisfaction, quality of life, and catheter-related complications in long-term urinary catheter users: a nationwide survey
- PMID: 40748488
- PMCID: PMC12316781
- DOI: 10.1007/s00345-025-05850-8
Patient satisfaction, quality of life, and catheter-related complications in long-term urinary catheter users: a nationwide survey
Abstract
Purpose: To compare patient satisfaction, quality of life, catheter-related complications between three types of catheterization in long-term urinary catheter users. To improve clinical decision-making for long-term urinary catheter users.
Methods: A nationwide survey study was conducted from August to September 2024. Patients who apply clean intermittent catheterization (CIC), have an urethral indwelling catheter (IDC), or a suprapubic catheter (SPC), were identified through the MediReva database, a Dutch medical supplier. The survey was developed by structured consensus meeting and consisted of the ICIq-LTCqol and the EQ-5D-5 L.
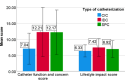
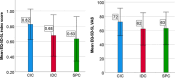
Results: 3320 patients participated in the study (response rate 33%). 2634 performed CIC, 383 had an IDC, and 303 had an SPC. 75.9% was male and the mean age was 72 years. CIC patients reported the best patient satisfaction and QoL scores. When corrected for multiple confounders IDC and SPC were independently associated with lower patient satisfaction and QoL scores. There was no difference in UTI incidence in the last 6 months between the groups.
Conclusions: This study shows differences in patient satisfaction, QoL and, catheter-related complications between three types of catheterization. Healthcare providers should be aware of the impact of bladder drainage methods on the patient satisfaction and QoL, especially for those using an IDC or SPC. This information can be of added value in the decision-making process of long-term bladder management.
Keywords: Clean intermittent catheterization; Indwelling catheterization; Lower urinary tract symptoms; Neurogenic bladder; Patient satisfaction; Quality of life.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Erasmus Medical Ethical Review Committee (MEC-2024-0303). Consent to participate: Informed consent was obtained from all individual participants included in the study.
Figures
References
-
- Selius BA, Subedi R (2008) Urinary retention in adults: diagnosis and initial management. Am Fam Physician 77(5):643–650 - PubMed
-
- Jamison J, Maguire S, McCann J (2013) Catheter policies for management of long term voiding problems in adults with neurogenic bladder disorders. Cochrane Database Syst Rev. 11:CD004375 - PubMed
-
- Sartori AM, Kessler TM, Castro-Díaz DM, de Keijzer P, Del Popolo G, Ecclestone H et al (2024) Summary of the 2024 update of the European association of urology guidelines on neurourology. Eur Urol 85(6):543–555 - PubMed
-
- Lapides J, Diokno AC, Silber SJ, Lowe BS (1971) Clean, intermittent self-catheterization in the treatment of urinary tract disease. Trans Am Assoc Genitourin Surg 63:92–96 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

