Increased Peripheral T Stem Cell-like Memory Features in Patients with Advanced Solid Tumors Treated with Tumor-Targeting IL-12 Immunocytokine Therapy
- PMID: 40748620
- PMCID: PMC12485384
- DOI: 10.1158/1078-0432.CCR-25-1490
Increased Peripheral T Stem Cell-like Memory Features in Patients with Advanced Solid Tumors Treated with Tumor-Targeting IL-12 Immunocytokine Therapy
Abstract
Purpose: T-cell stemness is important for antitumor immunity. The presence in tumor-draining lymph nodes and tumor of stem-like memory T (TSCM) cells and T cells expressing the transcription factor T-cell factor 1 (TCF1), critical in T-cell self-renewal, is associated with improved response to immune checkpoint inhibitors.
Experimental design: We studied the effects of the tumor-targeting immunocytokine PDS01ADC (NHS-IL12) on peripheral T-cell stemness in murine hosts and in patients with advanced solid tumors. TSCM and expression of the murine T-cell stemness markers stem cell antigen 1 (Ly6a) and Tcf7 were evaluated in naïve and tumor-bearing mice receiving murine PDS01ADC (NHS-muIL12). Peripheral blood from patients treated in a clinical trial with PDS01ADC was analyzed for TSCM and expression of TCF1 on T-cell subsets.
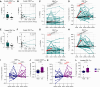
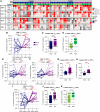
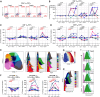
Results: NHS-muIL12 treatment in naïve mice increased stem cell antigen 1-positive peripheral T cells with stem-like phenotypes and promoted tumor infiltration of CD8+ T cells displaying increased stemness. In patients with advanced solid tumors, PDS01ADC treatment increased peripheral CD8+ and CD4+ TSCM frequencies and effector memory T cells expressing TCF1, with greater increases associated with disease control. Most peripheral TSCM cells were negative for PD-1 and TIGIT throughout treatment, suggesting their quiescence and self-renewal capacity. Expanded TCF1-negative effector memory CD8+ T cells expressed PD-1 with increased intensity of granzyme B, indicating an activated, cytotoxic state.
Conclusions: PDS01ADC treatment in patients with advanced solid tumors boosts peripheral T cells with stem-like characteristics, correlating with disease stabilization. Further studies combining PDS01ADC with other immunotherapies to synergize with this peripheral burst of T-cell stemness are warranted.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
M.T. Lynch reports other support from PDS Biotechnology during the conduct of the study. J.L. Marté reports other support from PDS Biotechnology during the conduct of the study. J.L. Gulley reports other support from PDS Biotechnology during the conduct of the study. J. Schlom reports other support from PDS Biotechnology during the conduct of the study. R.N. Donahue reports other support from PDS Biotechnology during the conduct of the study. Per the above, the National Cancer Institute has a Cooperative Research and Development Agreement with PDS Biotechnology. No disclosures were reported by the other authors.
Figures


References
-
- Ren Z, Zhang X, Fu YX. Facts and hopes on chimeric cytokine agents for cancer immunotherapy. Clin Cancer Res 2024;30:2025–38. - PubMed
-
- Sharifi J, Khawli LA, Hu P, King S, Epstein AL. Characterization of a phage display-derived human monoclonal antibody (NHS76) counterpart to chimeric TNT-1 directed against necrotic regions of solid tumors. Hybrid Hybridomics 2001;20:305–12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

