The Structure of Human IAPP Fibrils Reflects Membrane and pH Conditions
- PMID: 40748680
- PMCID: PMC12356532
- DOI: 10.1021/jacs.5c06971
The Structure of Human IAPP Fibrils Reflects Membrane and pH Conditions
Abstract
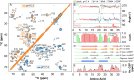
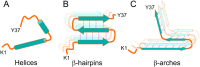
Physiologically relevant in vitro models of amyloid aggregation are essential for linking structural insights to disease pathology. In type 2 diabetes, aggregation of human islet amyloid polypeptide (hIAPP) into fibrils is a hallmark of β-cell dysfunction, yet structural data on ex vivo hIAPP fibrils remain unavailable. Most models use solution-grown fibrils, overlooking membrane interactions and native pH, which underscores the need for more realistic in vitro models. Here, we use solid-state NMR spectroscopy to determine the structure of phospholipid membrane-mediated hIAPP fibrils formed under extracellular (pH 7.4) conditions. These fibrils are homogeneous and adopt an L-shaped protofilament architecture with an extended N-terminal β-strand─a region often unresolved in cryo-EM. The fibril core (N14-L27) adopts the CF1 fold, a conserved β-arch also seen in nonlipidic fibrils, suggesting its relevance in disease. In contrast, fibrils formed at intracellular pH (5.3) are structurally heterogeneous and show distinct structural differences in the C-terminus. hIAPP must exhibit substantial structural plasticity in the membrane environment, transitioning from helical monomers to β-hairpin oligomers and ultimately to β-arch-rich fibrils─transitions that may introduce energy barriers stabilizing toxic intermediates. Our findings provide the first high-resolution structure of membrane-mediated hIAPP fibrils highlighting the need to model aggregation under physiologically relevant conditions.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

