Seven-Year Results for RESILIA Tissue in Bicuspid Aortic Valve Replacement Patients: Age and Valve Size Considerations
- PMID: 40748697
- PMCID: PMC12342796
- DOI: 10.1093/icvts/ivaf176
Seven-Year Results for RESILIA Tissue in Bicuspid Aortic Valve Replacement Patients: Age and Valve Size Considerations
Abstract
Objectives: Patients with bicuspid aortic valve disease requiring surgical aortic valve replacement are often younger and want to avoid lifelong anticoagulation. A multicentre single-arm non-randomized study, the COMMENCE trial, studied outcomes of RESILIA tissue aortic valves in bicuspid aortic valve patients through 7 years of follow-up.
Methods: Of 672 patients who underwent surgical replacement of native aortic valves, 214 had bicuspid and 458 had tricuspid aortic valves. Propensity score analyses with inverse probability of treatment weighting were utilized to minimize bias due to measured confounders. Linear mixed-effect models compared longitudinal changes in haemodynamic parameters.
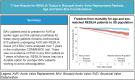
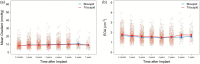
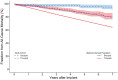
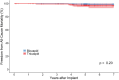
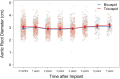
Results: Patients with bicuspid were significantly younger than those with tricuspid aortic valves-mean age of bicuspid: 59.8 (12.4) vs tricuspid: 70.2 (9.5) years; P < .001; 39/214 (18%) bicuspid aortic valve patients were <50 years old. There was no evidence of structural valve deterioration in any bicuspid aortic valve patients over 7 years of follow-up. At 7 years, there was no significant difference between bicuspid and tricuspid aortic valve patients in propensity score- and age-adjusted survival (91.9% vs 88.1%, respectively; P = .35), stroke, or reoperation. Among bicuspid aortic valve patients <65 years of age, there was no significant difference in prosthetic valve effective orifice areas and mean gradients between 3 months and 7 years postoperatively.
Conclusions: Patients with bicuspid aortic valves had excellent outcomes with RESILIA tissue valves at 7 years with no evidence of structural valve deterioration. These results suggest a durable alternative for carefully selected younger patients wishing to avoid anticoagulation.
Clinical trial registration number: NCT01757665.
Keywords: RESILIA; aortic regurgitation; aortic stenosis; aortic valve replacement; bicuspid aortic valve; clinical outcomes.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery.
Conflict of interest statement
J.E.B.: Consultant for Edwards Lifesciences, Abbott, Medtronic, Terumo Aortic, Artivion. T.B.: Atricure (consultant), Edwards Lifesciences (consultant), Nexus (DSMB). P.P.: Institutional research funding from Cardiac Success, Edwards Lifesciences, Medtronic, Novartis, Pi-Cardia. M.A.B.: Consultant to Abbott, Artivion, Edwards Lifesciences, Medtronic. V.H.T.: Equity: Dasi Simulations; Consulting or research: Abbott Vascular, Artivion, Atricure, Boston Scientific, Croivalve, Edwards Lifesciences; Highlife; Innovalve; Jenavalve, Medtronic, Trisol.
Figures
References
-
- Yang LT, Ye Z, Ullah MW, et al. Bicuspid aortic valve: long-term morbidity and mortality. Eur Heart J. 2023;44:4549-4562. - PubMed
-
- Otto CM, Newby DE. Transcatheter valve replacement for bicuspid aortic stenosis. JAMA. 2021;326:1009-1010. - PubMed
-
- Iribarne A, Leavitt BJ, Robich MP, et al. ; Northern New England Cardiovascular Disease Study Group. Tissue versus mechanical aortic valve replacement in younger patients: a multicenter analysis. J Thorac Cardiovasc Surg. 2019;158:1529-1538.e2. - PubMed
-
- Mahjoub H, Mathieu P, Larose E, et al. Determinants of aortic bioprosthetic valve calcification assessed by multidetector CT. Heart. 2015;101:472-477. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

