A bacteria-based search for drugs against avian and swine flu yields a potent and resistance-resilient channel blocker
- PMID: 40748960
- PMCID: PMC12337319
- DOI: 10.1073/pnas.2502240122
A bacteria-based search for drugs against avian and swine flu yields a potent and resistance-resilient channel blocker
Abstract
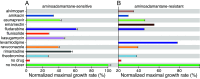
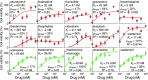
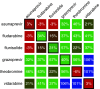
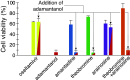
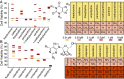
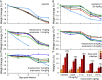
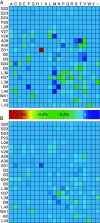
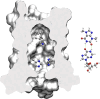
Influenza represents a significant threat with seasonal epidemics that can transition to global pandemics, and cross-species infection presenting a continuous challenge. While vaccines and several antiviral drugs are available, constant genetic changes vitiate these prevention and treatment options. Consequently, we decided to search for inhibitors against one of the virus's validated drug targets, its M2 channel that is blocked by aminoadamantanes. Regrettably, widespread mutations in M2 abolish the antiflu activity of said blockers. Therefore, we devised bacteria-based genetic assays that can screen for drugs against aminoadamantane-sensitive and resistant M2 channels and map the resistance potential of any identifiable blocker. Subsequent in cellulo testing and structure-activity relationship studies yielded a synergistic combination of two compounds, Theobromine and Arainosine, that exhibited remarkable antiviral activity by directly inhibiting the virus's channel. The drug duo was potent against H1N1 pandemic swine flu, H5N1 pandemic avian flu, and aminoadamantane-resistant and sensitive strains alike, exhibiting activity that surpassed oseltamivir, the leading antiflu drug on the market. When this drug duo was tested in an animal model, it once more outperformed oseltamivir, considerably reducing disease symptoms and viral RNA progeny. Importantly, harnessing the bacterial genetic selection, we could demonstrate that the drug duo's potential for eliciting drug resistance is significantly smaller and molecularly distinct from that of aminoadamantanes. In conclusion, the outcome of this study represents a new potential treatment option for influenza alongside an approach that is sufficiently general and readily applicable to other viral targets.
Keywords: channel blockers; influenza; ion channel.
Conflict of interest statement
Competing interests statement:I.T.A. has stock in a company that is trying to commercialize some of the results of the study. H.L. and I.T.A. have filed for a patent for the anti-influenza work.
Figures

Similar articles
-
Potent Anti-Influenza Synergistic Activity of Theobromine and Arainosine.bioRxiv [Preprint]. 2024 Oct 15:2024.10.13.618054. doi: 10.1101/2024.10.13.618054. bioRxiv. 2024. PMID: 39416015 Free PMC article. Preprint.
-
Repurposing of Some Nucleoside Analogs Targeting Some Key Proteins of the Avian H5N1 Clade 2.3.4.4b to Combat the Circulating HPAI in Birds: An In Silico Approach.Viruses. 2025 Jul 10;17(7):972. doi: 10.3390/v17070972. Viruses. 2025. PMID: 40733589 Free PMC article.
-
In vitro antiviral activities of thymol and Limonin against influenza a viruses and SARS-CoV-2.Sci Rep. 2025 Jul 2;15(1):22587. doi: 10.1038/s41598-025-06967-x. Sci Rep. 2025. PMID: 40596436 Free PMC article.
-
Systematic review of influenza resistance to the neuraminidase inhibitors.BMC Infect Dis. 2011 May 19;11:134. doi: 10.1186/1471-2334-11-134. BMC Infect Dis. 2011. PMID: 21592407 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

