Anion-Exchange-Membrane Electrolysis with Alkali-Free Water Feed
- PMID: 40748968
- PMCID: PMC12355722
- DOI: 10.1021/acs.chemrev.4c00466
Anion-Exchange-Membrane Electrolysis with Alkali-Free Water Feed
Abstract
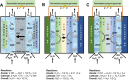
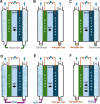
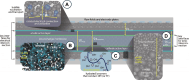
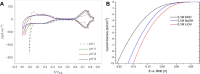
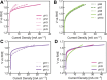
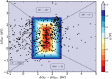
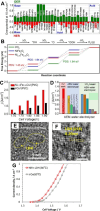
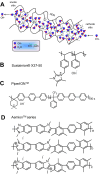
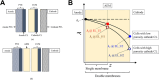
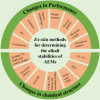
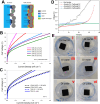
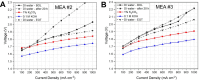
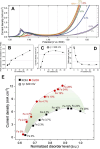
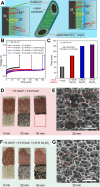
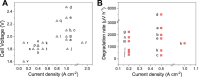
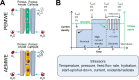
Hydrogen is a green and sustainable energy vector that can facilitate the large-scale integration of intermittent renewable energy, renewable fuels for heavy transport, and deep decarbonization of hard-to-abate industries. Anion-exchange-membrane water electrolyzers (AEM-WEs) have several achieved or expected competitive advantages over other electrolysis technologies, including the use of precious metal-free electrocatalysts at both electrodes, fluorine-free hydrocarbon-based ionomeric membranes and bipolar plates based on inexpensive materials. Contrasting the analogous proton-exchange-membrane system (PEM-WE), where pure water is circulated (no support electrolyte), the current generation of AEM-WEs necessitates the circulation of a dilute aqueous alkaline electrolyte for reaching high energy efficiency and durability. For several reasons, including but not limited to lower cost of balance-of-plant, lower operating cost and improved device's lifetime, achieving high cell efficiency and performance using an alkali-free water feed is highly desirable. In this review, we develop and build a foundational understanding of AEM-WEs operating with pure water, as well as discuss the effects of operating with natural water feeds like seawater. After a discussion of the possible advantages of pure-water-fed AEM-WEs, we cover the thermodynamic and kinetic processes involved in AEM-WE, followed by a detailed review of materials and components and their integration in the device. We highlight the influence of electrolyte composition and alkali/electrolyte-free feed on the membrane-electrode assembly, ionomers, electrocatalysts, porous transport layer, bipolar plates and operating configuration. We provide evidence for how the pure water feed engenders several issues related to the degradation of device components and propose mitigation strategies.
Figures










Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Enhanced NiFe2O4 Catalyst Performance and Stability in Anion Exchange Membrane Water Electrolysis: Influence of Iron Content and Membrane Selection.Molecules. 2025 Aug 1;30(15):3228. doi: 10.3390/molecules30153228. Molecules. 2025. PMID: 40807402 Free PMC article.
-
Cost-Effective Bimetallic Catalysts for Green H2 Production in Anion Exchange Membrane Water Electrolyzers.Nanomaterials (Basel). 2025 Jul 4;15(13):1042. doi: 10.3390/nano15131042. Nanomaterials (Basel). 2025. PMID: 40648747 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- IEA . Net Zero by 2050 - A Roadmap for the Global Energy Sector.
-
- Kovač A., Paranos M., Marciuš D.. Hydrogen in Energy Transition: A Review. Int. J. Hydrogen Energy. 2021;46(16):10016–10035. doi: 10.1016/j.ijhydene.2020.11.256. - DOI
-
- Fonseca J. D., Camargo M., Commenge J.-M., Falk L., Gil I. D.. Trends in Design of Distributed Energy Systems Using Hydrogen as Energy Vector: A Systematic Literature Review. Int. J. Hydrogen Energy. 2019;44(19):9486–9504. doi: 10.1016/j.ijhydene.2018.09.177. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

