Identification of the Francisella novicida FTN_0096 as a factor involved in intracellular replication and host response
- PMID: 40748985
- PMCID: PMC12316277
- DOI: 10.1371/journal.pone.0329626
Identification of the Francisella novicida FTN_0096 as a factor involved in intracellular replication and host response
Abstract
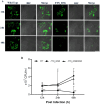
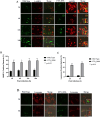
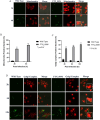
Francisella tularensis is the causative agent of the zoonotic disease tularemia. We investigated a pathogenic factor of F. tularensis subsp. novicida (F. novicida). Accordingly, we established a novel infection model using HeLa cells. F. novicida usually infects macrophage lineage cells and less frequently epithelial cells. We successfully infected HeLa cells expressing the Fc receptor (HeLa-FcγRII cells) using F. novicida supplemented with mouse serum containing F. novicida antibodies. A total of 2,232 transposon mutants of F. novicida were screened to determine the relatively fewer cytotoxic strains of the HeLa-FcγRII cells, and 13 strains were thus isolated. Sequencing analysis of transposon insertion sites identified 13 genes, including FTN_0096. We focused on FTN_0096. Although the F. novicida wild-type strain proliferated in HeLa-FcγRII and THP-1 cells, the number of intracellular FTN_0096 mutant decreased. FTN_0096 mutant cannot escape from phagolysosomes in the initial phases of infection. Moreover, FTN_0096 mutant was detected in the mitochondria and Golgi complex. These findings indicate the importance of FTN_0096 of F. novicida for intracellular replication in the cells.
Copyright: © 2025 Wardhana et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
A novel method of Francisella infection of epithelial cells using HeLa cells expressing fc gamma receptor.BMC Infect Dis. 2024 Oct 17;24(1):1171. doi: 10.1186/s12879-024-10083-y. BMC Infect Dis. 2024. PMID: 39420255 Free PMC article.
-
TolC and EmrA1 contribute to Francisella novicida multidrug resistance and modulation of host cell death.J Bacteriol. 2024 Sep 19;206(9):e0024624. doi: 10.1128/jb.00246-24. Epub 2024 Aug 28. J Bacteriol. 2024. PMID: 39194223 Free PMC article.
-
RtxA like protein contributes to infection of Francisella novicida in silkworm and human macrophage THP-1.Microb Pathog. 2018 Oct;123:74-81. doi: 10.1016/j.micpath.2018.06.046. Epub 2018 Jun 30. Microb Pathog. 2018. PMID: 29969671
-
Comparative review of Francisella tularensis and Francisella novicida.Front Cell Infect Microbiol. 2014 Mar 13;4:35. doi: 10.3389/fcimb.2014.00035. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24660164 Free PMC article. Review.
-
Live Attenuated Tularemia Vaccines for Protection Against Respiratory Challenge With Virulent F. tularensis subsp. tularensis.Front Cell Infect Microbiol. 2018 May 15;8:154. doi: 10.3389/fcimb.2018.00154. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29868510 Free PMC article. Review.
References
-
- Petersen JM, Mead PS, Jameson JL, Fauci AS, Kasper DL, Hauser SL. Tularemia. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, editors. Harrison’s principles of internal medicine. 20e ed. New York, NY: McGraw-Hill Education; 2018. p. 1196–9.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

