Comparative performance of a commercial and in-house Mp1p antigen-detecting enzyme immunoassay for the rapid diagnosis of talaromycosis
- PMID: 40749024
- PMCID: PMC12342302
- DOI: 10.1371/journal.pntd.0013248
Comparative performance of a commercial and in-house Mp1p antigen-detecting enzyme immunoassay for the rapid diagnosis of talaromycosis
Abstract
Background: Several antigen-detection assays have been developed for the rapid diagnosis of talaromycosis, but their utility has been limited by a lack of commercial options. The aim of this study was to perform a head-to-head comparison of the performance of our in-house monoclonal antibody-based Mp1p antigen-detecting enzyme immunoassay (EIA) with its recently-developed commercial platform.
Methods: In this diagnostic accuracy, retrospective, case-cohort study, we compared the sensitivity, specificity, positive likelihood ratio (LR+) and negative likelihood ratio (LR-) of the commercial Wantai Mp1p EIA versus our in-house Mp1p EIA on paired plasma and urine samples from 424 hospitalized adults with advanced HIV disease, including 224 cases of proven talaromycosis, where Talaromyces marneffei was isolated in culture of blood or other clinical specimens, and 200 controls diagnosed with a range of other opportunistic infections. All participants were randomly selected from prospective cohorts recruited between 2011 and 2019 from five centers in Vietnam.
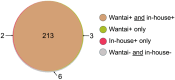
Results: The sensitivity of the Wantai and in-house Mp1p EIAs were comparable in plasma (95.1% vs 92.4%, P = 0.11), in urine (91.5% vs 87.1% P = 0.07), and in combined testing of plasma and urine (96.4% vs 96.0%, P = 1.00), where talaromycosis was diagnosed based on the positivity of either specimen. The specificity of the Wantai and in-house Mp1p EIAs were consistently high in plasma, in urine, and in combined testing (93 - 97%). The Wantai and in-house Mp1p EIAs provided substantially higher sensitivity than blood culture detection (96.4% and 96.0% vs 78.6%, P < 0.001). For both EIAs, LR+ were greater than 10 and LR- were less than 0.1, which increases the confidence to rule in or rule out talaromycosis.
Conclusions: The diagnostic performance of the Wantai Mp1p EIA was comparable to our validated in-house Mp1p EIA, and significantly more sensitive than blood culture, offering a standardized tool for the rapid diagnosis of talaromycosis.
Copyright: © 2025 Barwatt et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: TL has received investigator-initiated research grant from Gilead Science. No other authors disclose any competing interestings.
Figures



References
-
- Morris AJ, Kim HY, Nield B, Dao A, McMullan B, Alastruey-Izquierdo A, et al. Talaromyces marneffei, Coccidioides species, and Paracoccidioides species-a systematic review to inform the World Health Organization priority list of fungal pathogens. Med Mycol. 2024;62(6):myad133. doi: 10.1093/mmy/myad133 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

