Dynamic regulation of Arabidopsis β-AMYLASE1 by glutathione and thioredoxins affects starch in guard cells
- PMID: 40749095
- PMCID: PMC12351283
- DOI: 10.1093/plphys/kiaf344
Dynamic regulation of Arabidopsis β-AMYLASE1 by glutathione and thioredoxins affects starch in guard cells
Abstract
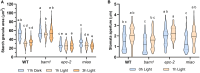
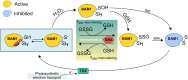
Guard cells control the opening and closure of stomatal pores in response to internal and external stimuli, ensuring gas exchange in plants. In Arabidopsis (Arabidopsis thaliana), β-AMYLASE1 (BAM1), assisted by α-AMYLASE3, begins degrading starch at dawn in guard cells to promote stomatal opening. Both enzymes are controlled by reversible disulfide bond formation, which decreases their activity. In the present study, we investigated the sensitivity of BAM1 to other redox-dependent post-translational modifications (PTM) both in vitro and in vivo. In vitro, H2O2 reversibly inactivates BAM1 and, in the presence of glutathione (GSH), induces S-glutathionylation of BAM1. Glutathionylated BAM1 is active and transiently protected from H2O2 inhibition. However, the glutathionylated state of BAM1 has limited stability and can be slowly resolved by a second cysteine with the formation of the intramolecular disulfide bond that inhibits BAM1 activity. Thioredoxin f can fully revert the inhibition by reducing the disulfide to a dithiol. In vivo, Arabidopsis mutants with lower plastidial GSH reductase activity, and consequently modified GSH homeostasis, showed higher BAM1 activity, lower starch levels in guard cells, and altered stomata aperture, indicating that GSH redox potential impacts stomatal physiology, possibly through BAM1. Moreover, plastidial BAM1 presents a prime example for the role of glutathionylation functioning as a transiently protective PTM, interfering with the formation of inhibitory disulfide bonds. This example illustrates how transitions between protein cysteinyl thiol PTMs can orchestrate dynamic responses involving several redox systems.
© The Author(s) 2025. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures





References
-
- Bohle F, Rossi J, Tamanna SS, Jansohn H, Schlosser M, Reinhardt F, Brox A, Bethmann S, Kopriva S, Trentmann O, et al. Chloroplasts lacking class I glutaredoxins are functional but show a delayed recovery of protein cysteinyl redox state after oxidative challenge. Redox Biol. 2024:69:103015. 10.1016/j.redox.2023.103015 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

