Proteogenomic characterization unveils biomarkers associated with chemoresistance in muscle-invasive bladder cancer
- PMID: 40749681
- PMCID: PMC12432383
- DOI: 10.1016/j.xcrm.2025.102255
Proteogenomic characterization unveils biomarkers associated with chemoresistance in muscle-invasive bladder cancer
Abstract
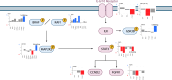
To explore potential chemoresistance mechanisms and identify therapeutic opportunities in muscle-invasive bladder cancer (MIBC), we conduct comprehensive proteogenomic characterization of 46 pre- and 14 post-treatment MIBC tumors incorporating genomics, transcriptomics, proteomics, and phosphoproteomics. Multi-omics clustering not only recapitulated established molecular subtypes but also revealed subtypes associated with chemotherapy sensitivity. Protein isoform level analysis identifies protein abundance of a short isoform of ATAD1 and RAF family proteins as biomarkers of chemosensitivity. Integration of proteomic and phosphoproteomic data reveals Wnt signaling via GSK3B-S9 phosphorylation and the JAK/STAT pathway as potential targets to overcome chemoresistance. Correlations between PD-L1 and TROP-2/NECTIN-4 indicate an additive benefit of combination therapy targeting these proteins. Overall, this study serves as a valuable resource for researchers and clinicians aiming to better understand and treat chemoresistant MIBC.
Keywords: GSK3B; RAF family; STAT3; biomarkers; chemoresistance; muscle-invasive bladder cancer; proteogenomics.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.E.D. is a compensated employee of StemMed Ltd. Some PDXs are exclusively licensed to StemMed Ltd., resulting in royalty income to L.E.D. P.D.C. is a partner of Sample-Kiosk. M.J.E. is the founder and shareholder of Progendis Inc (Consulting) and Bioclassifier LLC. Issued and licensing patents include Gene Expression Profiles to Predict Breast Cancer Outcomes, US pub # 20230250484, EP2297359AI. M.J.E.’s recent employment and current share options are with AstraZeneca. B.Z. received research funding from AstraZeneca and consulting fees from Inotiv. S.P.L. has funding for clinical trials from Aura Biosciences, FKD, JBL (SWOG), Genentech (SWOG), Merck (Alliance), QED Therapeutics, SURGE Therapeutics, Vaxiion, and Viventia; is a consultant/advisory board member for Aura Bioscience, BMS, Gilead, Incyte, Pfizer/EMD Serono, Protara, Surge Therapeutics, UroGen, Vaxiion, and Verity; has a patent for the TCGA classifier and received honoraria from Grand Rounds in Urology and UroToday; has stock options from Aura Biosciences; and received funds for stock options from C2I Genomics/Veracyte.
Figures






References
-
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration Adjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur. Urol. 2005;48:189–199. discussion 199–201. - PubMed
-
- Grossman H.B., Natale R.B., Tangen C.M., Speights V.O., Vogelzang N.J., Trump D.L., White R.W.d., Sarosdy M.F., Wood D.P., Jr., Raghavan D., Crawford E.D. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003;349:859–866. - PubMed
-
- Griffiths G., Hall R., et al. International Collaboration of Trialists. Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group) European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group. Australian Bladder Cancer Study Group. National Cancer Institute of Canada Clinical Trials Group. Finnbladder. Norwegian Bladder Cancer Study Group. Club Urologico Espanol de Tratamiento Oncologico Group International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J. Clin. Oncol. 2011;29:2171–2177. - PMC - PubMed
-
- Flaig T.W., Tangen C.M., Daneshmand S., Alva A., Lerner S.P., Lucia M.S., McConkey D.J., Theodorescu D., Goldkorn A., Milowsky M.I., et al. A Randomized Phase II Study of Coexpression Extrapolation (COXEN) with Neoadjuvant Chemotherapy for Bladder Cancer (SWOG S1314; NCT02177695) Clin. Cancer Res. 2021;27:2435–2441. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

