Clinical and metabolic phenotyping of continuous versus intermittent ENteral NUTrition in ventilated adults with shock: ENNUT trial protocol
- PMID: 40750277
- PMCID: PMC12315036
- DOI: 10.1136/bmjopen-2025-099761
Clinical and metabolic phenotyping of continuous versus intermittent ENteral NUTrition in ventilated adults with shock: ENNUT trial protocol
Abstract
Introduction: Critically ill patients often require enteral nutrition, but the optimal feeding strategy-continuous or intermittent-remains uncertain. While continuous enteral nutrition ensures steady nutrient delivery, it may inhibit key metabolic and cellular processes such as autophagy and ketogenesis. Intermittent enteral nutrition, by mimicking fasting periods, could activate protective pathways, potentially improving clinical outcomes. However, evidence for its efficacy and safety in intensive care units (ICUs) is limited. This study evaluates the clinical and metabolic impacts of fasting intervals during intermittent enteral nutrition compared with continuous enteral nutrition in critically ill patients.
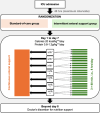
Methods and analysis: We designed a multicenter, randomised, open-label trial across nine French ICUs, enrolling adult patients requiring mechanical ventilation and vasopressor support. Participants will receive either intermittent or continuous enteral nutrition for 7 days, with the primary endpoint being the change in the Sequential Organ Failure Assessment (SOFA) score from Day 1 to Day 7 or ICU discharge. Secondary endpoints include nutritional intake, metabolic markers, gastrointestinal tolerance, ICU-acquired infections and mortality rates. Quality of life will be assessed at discharge. A total of 174 patients will be included. Descriptive statistics will summarise group characteristics, with the Student's t-test or the Mann-Whitney U test depending on data distribution for SOFA score change, and regression for confounders. Secondary endpoints will be analysed using regression, χ2 or Fisher's exact test, as appropriate.
Ethics and dissemination: The study protocol was approved by a French ethics committee on 24 October 2023 (Comité de Protection des Personnes Ile de France 1, Paris, France, number SI 23.03427.000435). Patients are included after providing informed consent. Results will be submitted for publication in a peer-reviewed journal.
Trial registration number: Registered on clinicaltrials.gov on 26 March 2023 (NCT06330610).
Keywords: Adult intensive & critical care; Intensive Care Units; Nutrition.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
