SRC Kinase Isoforms Regulate mRNA Splicing during Neural Development
- PMID: 40750357
- PMCID: PMC12369926
- DOI: 10.1523/JNEUROSCI.1705-24.2025
SRC Kinase Isoforms Regulate mRNA Splicing during Neural Development
Abstract
Alternative mRNA splicing generates transcriptomic diversity to direct tissue-specific functions. There is a high level of alternative splicing in the brain during embryonic development, but the master regulators of this process are poorly understood. One key splicing event in neuronal differentiation is the inclusion of a microexon in the SH3 domain of the ubiquitous tyrosine kinase, C-SRC, to yield the constitutively active, neural-specific N1-SRC kinase. We previously demonstrated that specific inhibition of N1-SRC in developing Xenopus embryos inhibits neurogenesis, but the targets and mode of action of N1-SRC were unknown. In the current study, we screened for N1-SRC SH3 domain interactors, surprisingly finding no unique targets compared with the C-SRC SH3 domain, but rather a subset of binding partners, enriched in splicing regulators. Analysis of public phosphoproteomic data revealed that SRC-dependent phosphorylation of the splicing machinery is widespread and enriched in RNA-binding proteins (RBPs). To investigate whether N1-SRC-dependent regulation of splicing underpins its role in neurogenesis, we undertook long- and short-read RNA-seq analysis of N1-SRC knockdown Xenopus embryos. We observed an upregulation of splicing factor expression and aberrant splicing of splicing regulators, principally HNRNPA1 and TRA2A. The affected splice junctions in both genes are in their glycine-rich C-termini, and junctions contain putative binding sites for SFPQ/NONO and FUS RBPs. Both SFPQ and FUS are SRC substrates, suggesting a mechanism by which N1-SRC knockdown leads to mis-splicing of HNRNPA1 and TRA2A. Thus, the neuronal splicing of C-SRC to generate N1-SRC regulates the alternative splicing landscape during neurogenesis.
Keywords: SRC; Xenopus; neurogenesis; phosphorylation; tyrosine kinase.
Copyright © 2025 Pizzey et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

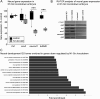



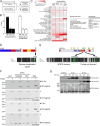
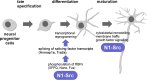
References
-
- Brunton VG, Avizienyte E, Fincham VJ, Serrels B, Metcalf CA 3rd, Sawyer TK, Frame MC (2005) Identification of Src-specific phosphorylation site on focal adhesion kinase: dissection of the role of Src SH2 and catalytic functions and their consequences for tumor cell behavior. Cancer Res 65:1335–1342. 10.1158/0008-5472.CAN-04-1949 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
