Robotic process automation to identify patients at high risk for perioperative myocardial infarction or injury: a prospective, blinded, paired reader-controlled single-centre study
- PMID: 40750466
- PMCID: PMC12597386
- DOI: 10.1016/j.bja.2025.07.035
Robotic process automation to identify patients at high risk for perioperative myocardial infarction or injury: a prospective, blinded, paired reader-controlled single-centre study
Abstract
Background: Although current guidelines recommend active surveillance for perioperative myocardial infarction, injury, or both in high-risk patients, implementation remains limited in most institutions worldwide because of a lack of resources.
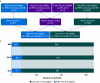
Methods: We hypothesised that robotic process automation (RPA), a software technology that enables virtual bots to replicate human tasks within digital systems, could accurately replace experienced clinical staff. Manual screening by experienced clinical staff and RPA screening were carried out simultaneously and blinded to identify high-risk patients eligible for active surveillance for myocardial infarction/injury, according to predefined screening criteria. Discrepant identification was reviewed by an independent clinician blinded to the origin of the identification, generating a reference standard classification of paired reader-controlled patients to investigate the primary diagnostic endpoint: relative true positive fraction.
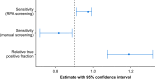
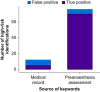
Results: In 660 participants (median age 60 yr, interquartile range 42-73 yr, 54.8% female), 77/660 (12%) were eligible for active surveillance for perioperative myocardial infarction or injury according to the reference standard classification. RPA screening achieved 75 (97%) true positive identifications, compared with 63 (82%) identified from manual screening (relative true positive fraction: 1.19, 95% confidence interval 1.08-1.32, P=0.004). The number needed to screen to identify one additional true positive using RPA screening was 6. RPA screening had a sensitivity of 0.97 (0.91-0.99), compared with 0.82 (0.72-0.89) for. Both approaches had high specificity (RPA screening: 0.98 [0.97-0.99], compared with manual screening: 1.0 [0.99-1.00]). The estimated annual cost of RPA screening was 81% lower compared with manual screening.
Conclusions: RPA screening was superior to standard-of-care manual screening by experienced clinical staff in identifying patients at high risk for perioperative myocardial infarction or injury.
Clinical trial registration: NCT02573532.
Keywords: active surveillance; myocardial injury after noncardiac surgery; perioperative care; perioperative myocardial infarction; perioperative myocardial injury; robotic process automation.
Copyright © 2025 British Journal of Anaesthesia. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of interest CM reports research grants from the Swiss Heart Foundation, the Swiss National Science Foundation, the University Hospital Basel, the University of Basel, Innosuisse, Abbott, AstraZeneca, Beckman Coulter, Boehringer Ingelheim, BRAHMS Thermo Fisher Scientific, Idorsia, LSI Medience Corporation, Novartis, Ortho Diagnostics, Quidel, Roche, Siemens, Singulex, SpinChip, Sphingotec, and speaker honoraria/consulting honoraria from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Idorsia, Novartis, Novo Nordisk, Osler, Roche, SpinChip, and Sanofi, all paid to the institution. CP reports grants from the Swiss Heart Foundation, Roche Diagnostics and the University Hospital Basel. DMG has received speaker or consulting honoraria from Roche, outside the submitted work. FM has been supported by Deutsche Forschungsgemeinschaft (SFB TRR219, Project-ID 322900939), and Deutsche Herzstiftung. Saarland University has received scientific support from Ablative Solutions, Medtronic, and ReCor Medical. Until May 2024, FM has received speaker honoraria/consulting fees from Ablative Solutions, Astra Zeneca, Inari, Medtronic, Merck, Novartis, Philips, and ReCor Medical. NG reports grants from the Swiss Heart Foundation. CP reports grants from the Swiss Heart Foundation, Roche Diagnostics and the University Hospital Basel dedicated to conduct of this study, and chaired an advisory board for Roche Diagnostics, during the conduct of the study. All other authors declare that they have no conflicts of interest.
Figures

References
-
- Nepogodiev D., Martin J., Biccard B., et al. Global burden of postoperative death. Lancet. 2019;393:401. - PubMed
-
- Puelacher C., Lurati Buse G., Seeberger D., et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137:1221–1232. - PubMed
-
- Botto F., Alonso-Coello P., Chan M.T.V., et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120:564–578. - PubMed
-
- Devereaux P.J., Chan M.T.V., Alonso-Coello P., et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304. - PubMed
-
- Beattie W.S., Karkouti K., Tait G., et al. Use of clinically based troponin underestimates the cardiac injury in non-cardiac surgery: a single-centre cohort study in 51,701 consecutive patients. Can J Anaesth. 2012;59:1013–1022. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

