Tumor control and immune activation through palliative irradiation and ATR inhibition, PATRIOT Part C: a phase Ib trial
- PMID: 40750587
- PMCID: PMC12316918
- DOI: 10.1038/s41467-025-62249-0
Tumor control and immune activation through palliative irradiation and ATR inhibition, PATRIOT Part C: a phase Ib trial
Abstract
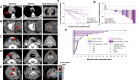
Ataxia telangiectasia and Rad3-related kinase (ATR) is a rational radiosensitization target. In this study, we explore the combination of the ATR inhibitor, ceralasertib, and palliative radiotherapy, with primary endpoint the identification of maximum tolerated dose, and secondary endpoints the determination of adverse event causality, pharmacokinetics (PK) and anti-tumor activity. Twenty-seven patients were dosed in escalating dose cohorts from 20 to 80 mg twice daily (BD) with concomitant radiation, 20 Gy in 10 fractions or 30 Gy in 15 fractions. Patients were assessed for acute and late toxicities and response after therapy. A non-tolerated dose was not reached. Maximum administered dose was 80 mg BD ceralasertib over 3 weeks with 30 Gy in 15 fractions, at which 1/6 evaluable patients had dose-limiting toxicities (radiation dermatitis and mucositis). PK was comparable to monotherapy. Of 23 efficacy-evaluable participants, 2 (9%) had complete response (CR), 6 (26%) partial response (PR), 13 (57%) stable disease (SD) and 2 (9%) progressive disease (PD) as best response in irradiated tumors. Response was not clearly linked to genomic aberrations. Increased T and natural killer cell activation as observed in peripheral blood as treatment progressed.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: K.E.S is an employee of The Institute of Cancer Research, which is involved in the development of PI3K, HSP90, HDAC, AKT, ROCK, RAF, CHK1, and HSF1 inhibitors. C.J.L. makes the following disclosures: receives and/or has received research funding from: AstraZeneca, Merck KGaA, Artios, Neophore. Received consultancy, SAB membership or honoraria payments from: Syncona, Sun Pharma, Gerson Lehrman Group, Merck KGaA, Vertex, AstraZeneca, Tango, 3rd Rock, Ono Pharma, Artios, Abingworth, Tesselate, Dark Blue Therapeutics, Pontifax, Astex, Neophore, Glaxo Smith Kline, Dawn Bioventures. Has stock in: Tango, Ovibio, Hysplex, Tesselate. U.B. reports research funding, honoraria and advisory board membership for Chugai Pharmaceutical, Verastem Inc., and Carrick Therapeutics K.J.H. reports honoraria (inst.): ALX Pharma, Arch Oncology, AstraZeneca, Bicara Therapeutics, BMS, Boehringer Ingelheim, Codiak Biosciences, F-Star Therapeutics, Inzen Therapeutics, Merck Serono, Merus, MSD, Oncolys Biopharma, Pfizer, Replimune, VacV Biotherapeutics; Consulting or Advisory Role (inst.): Arch Oncology, AstraZeneca, BMS, Boehringer Ingelheim, Inzen Therapeutics, Merck Serono, MSD, Oncolys BioPharma, Replimune; Speakers’ Bureau (inst.): BMS, Merck Serono, MSD; Research Funding (inst.): AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Replimune. E.D., S.A.S., N.S. are employees of AstraZeneca with stock ownership. The remaining authors declare no competing interests.
Figures




References
-
- Dillon, M. T., Good, J. S. & Harrington, K. J. Selective targeting of the G2/M cell cycle checkpoint to improve the therapeutic index of radiotherapy. Clin. Oncol. (R. Coll. Radio.)26, 257–265 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

