Lung structural cells are altered by infeluenza virus leading to rapid immune protection following re-challenge
- PMID: 40750594
- PMCID: PMC12317152
- DOI: 10.1038/s41467-025-62364-y
Lung structural cells are altered by infeluenza virus leading to rapid immune protection following re-challenge
Abstract
Lung structural cells form barriers against pathogens and trigger immune responses following infections. This leads to the recruitment of innate and adaptive immune cells some of which remain within the lung and contribute to enhanced pathogen control following subsequent infections. There is growing evidence that structural cells also display long-term changes following infection. Here we investigate long-term changes to mouse lung epithelial cells, fibroblasts, and endothelial cells following influenza virus infection finding that all three cell types maintain an imprint of the infection, particularly in genes linked to communication with T cells. MHCI and MHCII proteins continue to be expressed at higher levels in both differentiated epithelial cells and progenitor populations and several differentially expressed genes are downstream of the transcription factor, SpiB, a known orchestrator of antigen presentation. Lung epithelial cells from influenza-infected mice display functional changes, more rapidly controlling influenza virus than cells from naïve animals. This rapid anti-viral response and increased expression of molecules required to communicate with T cells demonstrates sustained and enhanced functions following infection. These data suggest lung structural cells display characteristics of immune memory which could affect outcomes that are protective in the context of infection or pathogenic in chronic inflammatory disorders.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
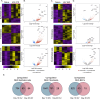



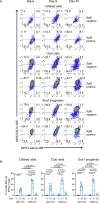

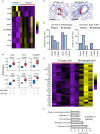

References
-
- Nair, H. et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet378, 1917–1930 (2011). - PubMed
-
- Somes, M. P., Turner, R. M., Dwyer, L. J. & Newall, A. T. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: a systematic review and meta-analysis. Vaccine36, 3199–3207 (2018). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

