A randomized, double-blind, placebo-controlled trial of niclosamide nanohybrid for the treatment of patients with mild to moderate COVID-19
- PMID: 40750596
- PMCID: PMC12317003
- DOI: 10.1038/s41467-025-62423-4
A randomized, double-blind, placebo-controlled trial of niclosamide nanohybrid for the treatment of patients with mild to moderate COVID-19
Abstract
Effective and reliable treatments for SARS-CoV-2 infections are a key part of global COVID-19 management. Based on vitro studies, niclosamide has been considered as a potential drug candidate for SARS-CoV-2, but its clinical development has been limited due to poor solubility and bioavailability. Here we report results from a randomized, double-blind, placebo-controlled clinical trial involving 300 patients (Clinical Trial Registration Number: KCT0007307) that assessed the efficacy and safety of the niclosamide nanohybrid CP-COV03 at two different doses. Oral CP-COV03 was well tolerated, with no serious adverse events reported in any treatment group. The primary endpoints demonstrated that CP-COV03 significantly alleviated all 12 FDA-recommended COVID-19 symptoms, with symptom improvement sustained for more than 48 h. Additionally, CP-COV03 reduced SARS-CoV-2 viral load by 56.7% within 16 h of the initial dose compared to baseline. Secondary endpoints, including time to sustained symptom resolution, time to return to usual health, and reduction in hospitalization risk, also showed favorable results in the CP-COV03 group compared to placebo. These findings indicate that CP-COV03 is a safe and effective therapeutic option for the treatment of mild to moderate COVID-19 and represents a promising advancement in the repurposing of niclosamide through nanohybrid engineering.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

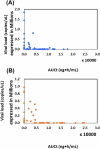
Similar articles
-
Nirmatrelvir-ritonavir versus placebo-ritonavir in individuals with long COVID in the USA (PAX LC): a double-blind, randomised, placebo-controlled, phase 2, decentralised trial.Lancet Infect Dis. 2025 Aug;25(8):936-946. doi: 10.1016/S1473-3099(25)00073-8. Epub 2025 Apr 3. Lancet Infect Dis. 2025. PMID: 40188838 Clinical Trial.
-
SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19.Cochrane Database Syst Rev. 2021 Sep 2;9(9):CD013825. doi: 10.1002/14651858.CD013825.pub2. Cochrane Database Syst Rev. 2021. PMID: 34473343 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Nirmatrelvir combined with ritonavir for preventing and treating COVID-19.Cochrane Database Syst Rev. 2022 Sep 20;9(9):CD015395. doi: 10.1002/14651858.CD015395.pub2. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2023 Nov 30;11:CD015395. doi: 10.1002/14651858.CD015395.pub3. PMID: 36126225 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

