Healthcare effects and evidence robustness of reimbursable digital health applications in Germany: a systematic review
- PMID: 40750660
- PMCID: PMC12317029
- DOI: 10.1038/s41746-025-01879-6
Healthcare effects and evidence robustness of reimbursable digital health applications in Germany: a systematic review
Abstract
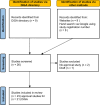
In Germany, statutory health insurance reimburses digital health applications (DiGAs) approved by the Federal Institute for Drugs and Medical Devices (BfArM). Permanent approval requires evidence of positive healthcare effects, either medical benefits or patient-relevant improvements in structures and processes (pSVV). This systematic review analyzed all 23 DiGA approval studies available as of March 15, 2024. The studies, conducted between 2012 and 2022, included 56 to 1245 participants and intervention durations from 1.5 to 12 months. Drop-out rates varied (mean 21.7% in intervention, 11.8% in control group). Most studies (13/23) focused on primary outcomes related to mental health conditions; one addressed pSVV. All reported significant medium to large effects, but risk of bias was high, particularly in outcome measurement (21/23) and due to missing data (15/23). These findings raise concerns about the robustness of the evidence supporting DiGA efficacy. The review recommends revising the DiGA approval process to enhance study quality. The systematic review was registered prospectively with PROSPERO https://www.crd.york.ac.uk/prospero/ (CRD42023460497).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: K.S., M.S. and S.D. declare no competing interests. Unrelated to this study, J.S. reports institutional grants for investigator-initiated research from the German G.B.A., B.M.G., B.M.B.F. and E.U. Federal State of Saxony, Novartis, Sanofi, A.L.K. and Pfizer. He has also participated in advisory board meetings as a paid consultant for Sanofi, Lilly, and A.L.K. J.S. is a member of the Expert Council on Health and Care at the Federal Ministry of Health and a member of the government commission for modern and needs-based hospital care of the current German Coalition.
Figures



References
-
- Bundesministerium für Gesundheit. Digital-Gesetz (DigiG). BGBl. Teil I Nr. 39, 22.12.2023, S. 4118–4137. (Deutschland, 2023).
-
- Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM). Das Fast-Track-Verfahren für digitale Gesundheitsanwendungen (DiGA) nach § 139e SGB V- DiGA Leitfaden, Version 3.5. (Bundesministerium für Gesundheit, 2023).
-
- Bundesministerium für Gesundheit. Gesetz für eine bessere Versorgung durch Digitalisierung und Innovation (Digitale-Versorgung-Gesetz – DVG, 2019).
-
- Gemeinsamer Bundesausschuss (G-BA). Nutzenbewertung von Arzneimitteln gemäß § 35a SGB V. https://www.g-ba.de/themen/arzneimittel/arzneimittel-richtlinie-anlagen/...
-
- Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM). DiGA-Verzeichnis. https://diga.bfarm.de/de/verzeichnis (2025).
Grants and funding
LinkOut - more resources
Full Text Sources

