NRAC controls CD36-mediated fatty acid uptake in adipocytes and lipid clearance in vivo
- PMID: 40750702
- PMCID: PMC12436663
- DOI: 10.1038/s44318-025-00520-2
NRAC controls CD36-mediated fatty acid uptake in adipocytes and lipid clearance in vivo
Abstract
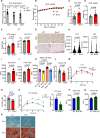
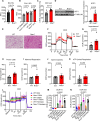
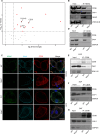
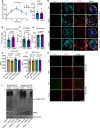
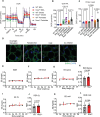
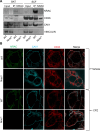
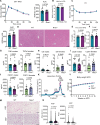
Adipose tissue is a central organiser of systemic lipid homeostasis and a pharmacological target in obesity, orchestrating cellular responses to environmental cues. Nutritionally regulated adipose and cardiac enriched protein (NRAC) is a small adipocyte-specific transmembrane protein with unknown function. Here, we show that Nrac directly interacts with scavenger receptor CD36 via its first transmembrane domain. Forming a complex with CD36 and caveolin-1 under low extracellular fatty acid (FA) concentrations, NRAC modulates CD36-dependent fatty acid uptake in adipocytes. Upon increase in extracellular FA levels, NRAC is ubiquitinated and internalised, leading to CD36's dissociation from caveolin-1 and clathrin-mediated endocytosis. This results in increased fatty acid uptake into fat cells, adipocyte hypertrophy, increased fat mass and elevated lipid clearance from the blood in chow-diet-fed mice. Finally, human NRAC expression and the intronic SNP rs12878589 are associated with body fat distribution and obesity. Together, these findings reveal a novel regulatory mechanism by which adipocytes sense and respond to extracellular fatty acid availability to fine-tune lipid uptake and storage at cellular and organismal level.
Keywords: Adipose Tissue; CD36; Clathrin-mediated Endocytosis; Fatty Acid Uptake; Hypertrophy.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. MB received honoraria as a consultant and speaker from Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Lilly, Novo Nordisk, Novartis, and Sanofi. All other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


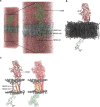
References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2:19–25
-
- Bakker AH, Nijhuis J, Buurman WA, van Dielen FM, Greve JW (2006) Low number of omental preadipocytes with high leptin and low adiponectin secretion is associated with high fasting plasma glucose levels in obese subjects. Diab Obes Metab 8:585–588 - PubMed
-
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C et al (2011) Brown adipose tissue activity controls triglyceride clearance. Nat Med 17:200–205 - PubMed
MeSH terms
Substances
Grants and funding
- 201830047/Uehara Memorial Foundation (UMF)
- 01KX1012/Federal Ministry of Education and Research
- 209933838/Deutsche Forschungsgemeinschaft (DFG)
- 82DZD00601/Deutsches Zentrum für Diabetesforschung (DZD)
- 101120466/EC | Horizon Europe | Excellent Science | HORIZON EUROPE Marie Sklodowska-Curie Actions (MSCA)
LinkOut - more resources
Full Text Sources

