Canonical Wnt signaling regulates Mbd3 protein stability during neurogenesis
- PMID: 40750707
- PMCID: PMC12411611
- DOI: 10.1038/s12276-025-01510-4
Canonical Wnt signaling regulates Mbd3 protein stability during neurogenesis
Abstract
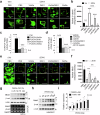
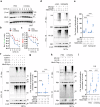
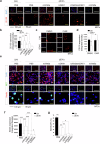
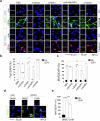
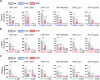
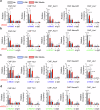
Acquisition of neural progenitor cell (NPC) homeostasis through balancing self-renewal and differentiation is essential for brain development and function. Among the mechanisms controlling these processes, canonical Wnt signaling and the Mbd3-NuRD complex, with prominent suppressive effects on neurogenesis, have been described as crucial parts of the core regulatory circuit. Here we explored Mbd3 as a downstream element of the canonical Wnt signalosomes. Specifically, dynamic modulation of Wnt signaling through activator (Wnt3a) and inhibitor (DKK1) resulted in parallel alterations in β-catenin and Mbd3 expression patterns. Also, overexpression and depletion of GSK3β respectively promoted and attenuated Mbd3 ubiquitination, highlighting that the canonical Wnt cascade promotes Mbd3 stability. Downstream of the Wnt-β-catenin pathway, Mbd3 represses transcription of neurogenesis-associated genes by triggering NuRD complex assembly, thereby promoting NPC stemness. This new Wnt-Mbd3 axis extends the current understanding of the canonical Wnt network in directing neuronal cell-fate determination in NPCs, suggesting this pathway as a potential target for driving neural stem cell reprogramming and neuronal lineage commitment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

