Valproic acid improves the efficacy of oxaliplatin/fluoropyrimidine-based chemotherapy by targeting cancer stem cell via β-Catenin modulation in colorectal cancer
- PMID: 40750758
- PMCID: PMC12316941
- DOI: 10.1038/s41419-025-07902-8
Valproic acid improves the efficacy of oxaliplatin/fluoropyrimidine-based chemotherapy by targeting cancer stem cell via β-Catenin modulation in colorectal cancer
Abstract
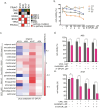
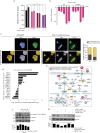
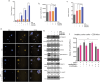
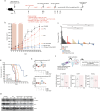
Despite advances in systemic therapeutic approaches, metastatic colorectal cancer (mCRC) patients harboring BRAF or RAS mutations have poor outcomes. Cancer stem cells (CSCs) play central roles in drug resistance and CRC recurrence. Therefore, targeting the epigenetic mechanisms that sustain CSC properties is a promising therapeutic approach. In this study, we report the efficacy of a treatment strategy with the potential to overcome chemotherapy resistance that involves administering the well-known antiepileptic drug and epigenetic agent valproic acid (VPA) and the standard chemotherapy regimen of oxaliplatin/fluoropyrimidine to wild-type CSCs and CSCs with BRAF and RAS mutations in enriched primary spheroid cultures. Notably, we demonstrated that VPA plus chemotherapy was more effective than other epigenetic drug-chemotherapy combinations by inhibiting cell proliferation and clonogenic growth and by inducing apoptosis and DNA damage. Mechanistically, proteomic analysis demonstrated that VPA induced CSC differentiation through the critical target of VPA, β-Catenin. Indeed, VPA promoted the proteasome-dependent degradation of β-Catenin by enhancing its binding to the E2 ubiquitin-conjugating enzyme UBE2a, leading to marked reductions in nuclear and cytoplasmic β-Catenin levels and subsequently decreasing β-Catenin/TCF-LEF target promoter activation. These effects were confirmed in three in vivo CRC xenograft models, including a syngeneic CT26 immunocompetent mouse model, where VPA combined with oxaliplatin/capecitabine chemotherapy and anti-VEGF therapy, a standard first-line treatment for mCRC, significantly suppressed tumor growth and prolonged survival with minimal toxicity. Proteomic analysis of tumor tissues from in vivo CRC models confirmed the VPA-mediated downregulation of CSC markers and β-Catenin.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All methods were carried out in accordance with relevant guidelines and regulations. Sphere cells were kindly provided by the Stassi G Laboratory and isolated from CRC patients undergoing colon resection, in accordance with the ethical standards regarding Human Experimentation (authorization CE9/2015, Policlinico Paolo Giaccone, Palermo). Informed consent was obtained from all subjects involved in the study. All animal procedures were performed in accordance with ARRIVE guidelines [52] (European directive 2010/63/UE and Italian Law—Directive 2010/63/EU and DL 26/2014) and were approved by the appropriate institutional review board (no. 377/2018-PR).
Figures

References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. - PubMed
-
- Cervantes A, Adam R, Rosello S, Arnold D, Normanno N, Taieb J, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10–32. - PubMed
-
- Zhang Z, Ji J, Liu H. Drug repurposing in oncology: current evidence and future direction. Curr Med Chem. 2021;28:2175–94. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

