High-selective HDAC6 inhibitor alleviates bone marrow fibrosis through inhibiting collagen formation and extracellular matrix deposition
- PMID: 40750768
- PMCID: PMC12317122
- DOI: 10.1038/s41598-025-08384-6
High-selective HDAC6 inhibitor alleviates bone marrow fibrosis through inhibiting collagen formation and extracellular matrix deposition
Abstract
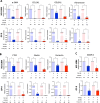
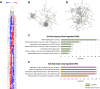
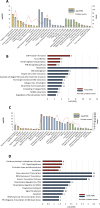
Bone marrow fibrosis (BMF) impairs normal hematopoietic functions in patients. The overactivation of the TGF-β signaling pathway is regarded as one of the offenders causing disease progression. Thus, factors capable of regulating TGF-β secretion hold great potential in reversing fibrotic diseases. One such factor is histone deacetylase inhibitors (HDACis), which can modulate the expression of TGF-β. Our previous study successfully synthesized a selective HDAC6 inhibitor, J22352, for pulmonary fibrosis; however, the treatment efficacies on BMF remain unclear. Therefore, in this study, we treated bone marrow-derived myofibroblasts with J22352. The results showed that J22352 significantly reduced cell viability, induced apoptosis, and inhibited extracellular matrix (ECM) accumulation. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis was employed to disclose potential mechanisms, identifying 334 differentially expressed proteins (DEPs). The DEPs were involved in cell apoptosis, programmed cell death, ECM deposition, and collagen formation. These results suggest that J22352 efficiently alleviated BMF by inducing cell apoptosis and inhibiting ECM deposition. This study introduces a novel selective HDAC6 inhibitor as a potential option for slowing down the progression of BMF. We aim to provide a promising selective HDACi for clinical medicine through a detailed analysis of its mechanisms and efficacy, offering new prospects in the field.
Keywords: Bone marrow fibrosis; Histone deacetylase inhibitor; Organ fibrosis; Transforming growth factor-β (TGF-β).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




References
-
- Kuter, D. J., Bain, B., Mufti, G., Bagg, A. & Hasserjian, R. P. Bone marrow fibrosis: Pathophysiology and clinical significance of increased bone marrow stromal fibres. Br. J. Haematol.139, 351–362. 10.1111/j.1365-2141.2007.06807.x (2007). - PubMed
-
- Schmitt, A. et al. Pathologic interaction between megakaryocytes and polymorphonuclear leukocytes in myelofibrosis. Blood96, 1342–1347 (2000). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

