Targeting AKR1B1 inhibits metabolic reprogramming to reverse systemic therapy resistance in hepatocellular carcinoma
- PMID: 40750772
- PMCID: PMC12317016
- DOI: 10.1038/s41392-025-02321-9
Targeting AKR1B1 inhibits metabolic reprogramming to reverse systemic therapy resistance in hepatocellular carcinoma
Abstract
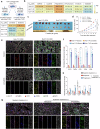
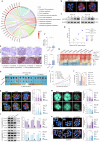
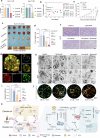
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality, and resistance to systemic therapies remains a significant clinical challenge. This study investigated the mechanisms by which metabolic reprogramming contributes to systemic treatment resistance in HCC. We established HCC cell lines with multidrug resistance characteristics and observed enhanced metabolic activity in these cells. Integrated multiomics analyses revealed hyperactive glucose‒lipid and glutathione metabolic pathways that play critical roles in supporting tumor cell proliferation and survival. We constructed a metabolic reprogramming atlas for HCC-resistant cells and identified aldo-keto reductase (Aldo-keto reductase family 1 Member B1, AKR1B1) as a key regulator of this reprogramming, which sustains drug resistance by regulating energy metabolism and enhancing stress tolerance. Importantly, AKR1B1 expression levels are closely associated with drug resistance and poor prognosis in HCC patients. The secretory nature of AKR1B1 not only underscores its predictive value but also facilitates the intercellular transmission of drug resistance. In terms of overcoming resistance, the AKR1B1 inhibitor epalrestat significantly mitigated drug resistance when it was used in combination with standard therapies. These findings underscore the importance of metabolic reprogramming in the development of HCC resistance. AKR1B1, a key enzyme that regulates metabolic reprogramming, has been identified as a potential biomarker and therapeutic target, providing new insights into overcoming resistance in HCC treatment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74, 229–263 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

