Synergic action of MicroRNAs and Wnts delivered by motor neuron EVs in promoting AChR clustering
- PMID: 40750900
- PMCID: PMC12315463
- DOI: 10.1186/s12964-025-02312-x
Synergic action of MicroRNAs and Wnts delivered by motor neuron EVs in promoting AChR clustering
Abstract
Background: The neuromuscular junction (NMJ) establishment occurs through complex communication events between motor neurons and muscle fibers; however, the molecular mechanisms leading to NMJ formation have yet to be fully elucidated. Little is known about the significance of extracellular vesicles (EVs) in mediating the interaction between motor neurons and muscle fiber in the NMJ establishment; this study investigates the role of motor neuron-derived EVs during the earliest stages of NMJ formation.
Methods: NSC-34 cells have been used as a model of motor neurons; EVs have been isolated during neurite development using a serial ultracentrifugation protocol specifically adjusted to isolate large and small EVs. Isolated EVs were quantified through Nanoparticles Tracking Assay and characterized by Western Blot and TEM analyses. The microRNA (miRNA) cargo of EV subpopulations was identified by small-RNA sequencing and the predicted miRNA downstream targets were investigated.
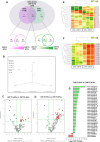
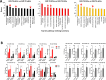
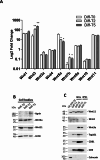
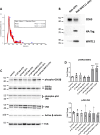
Results: NGS analysis of small RNAs carried by NSC-34-derived EVs identified a total of 245 EV specific miRNAs, most of which are up-regulated in NSC-34 cells and EVs during neurite stretching. Target prediction analysis evidenced how these miRNAs synergically target the Wnt signaling pathway. Moreover, we found that NSC-34-derived EVs carry Wnt proteins, including Wnt11, Wnt4 and Wnt3a. Since several studies suggested a role for the Wnt-associated signaling network in NMJ formation, we investigated the potential role of NSC-34 EVs in NMJ development and demonstrated that EV administration to myotubes increases acetylcholine receptor (AChR) cluster formation, as revealed by immunofluorescence staining with α-bungarotoxin. Moreover, myotube treatment with NSC-34-derived EVs led to GSK3β and JNK phosphorylation, followed by β-catenin nuclear translocation, suggesting that neuron-derived EVs can induce AChR clustering through Wnt pathway activation.
Conclusion: These data demonstrate that EVs released from differentiated motor neurons carry multimodal signals, miRNAs, and Wnts, which can stimulate AChR clustering in myotubes, a fundamental preparatory stage for NMJ formation. These new data highlight that EVs may play a role in the NMJ establishment and function under physiological and pathological conditions, particularly neurodegenerative diseases.
Keywords: Acetylcholine receptor; Agrin; Extracellular vesicles; Neuromuscular junctions; Wnt signalling; β-catenin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: All animal care and handling procedures were approved by the Institutional Animal Care and Use Committee of Istituto Italiano di Tecnologia, Genova, Italy. Consent for publication: All authors read and approved the final manuscript. Competing interests: The authors declare no competing interests.
Figures



References
-
- Li L, Xiong W-C, Mei L. Neuromuscular junction formation, aging, and disorders. Annu Rev Physiol. 2018;80:159–88. 10.1146/annurev-physiol-022516-034255. - PubMed
-
- Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. 10.1016/j.conb.2005.12.003. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

