Elderly individuals exhibit dysregulated monocyte responses to viral immune complexes compared to adults and children
- PMID: 40751004
- PMCID: PMC12316873
- DOI: 10.1038/s41598-025-13883-7
Elderly individuals exhibit dysregulated monocyte responses to viral immune complexes compared to adults and children
Abstract
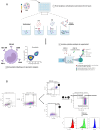
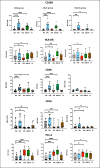
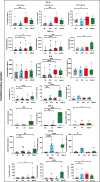
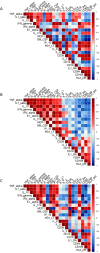
The severity of certain viral infectious diseases varies across the age; we hypothesize that these variations could be related to the variation of immune responses to viral immune complexes (ICs) among the age. This study aimed to investigate monocyte activation in response to ICs in children, adults, and elderly individuals. An experimental in vitro model was established using peripheral blood mononuclear cells from healthy individuals. Monocyte activation markers (CD169, CD38, HLA-DR), the negative co-stimulatory molecule (PD-L1), and cytokine production were measured under basal conditions and upon stimulation with human adenovirus 5-IgG immune complex (Ad5-ICs), interferon-alpha (IFN-α), and lipopolysaccharide (LPS). Monocytes from children and adults displayed similar activation profiles in response to ICs and IFN-α stimulation, characterized by increased expression of CD169 and PD-L1. In contrast, monocytes from elderly individuals exhibited weak or no overexpression of CD169 and PD-L1 coupled with a diminished PBMC cytokine response. Notably, cells from elderly participants produced high levels of TNF-α, IL-1α, and IL-6 in the absence of stimulation. Multiple comparisons confirmed reduced monocyte activation and PBMC cytokine responses in the elderly compared to adults and children. Although children exhibited a significant response to ICs, their secretion of IFN-α, IP-10, IFN-γ, IL-8, and IL-2 was lower than that observed in adults. Our findings suggest that elderly individuals have poor and dysregulated responses to ICs, likely due to immunosenescence and chronic inflammation. Adults exhibit a robust and balanced response to ICs, while children display a moderate response, possibly influenced by 'trained immunity' resulting from frequent early-life exposures to pathogens. These insights highlight the importance of further research to develop age-specific therapeutic strategies to modulate immune function during viral IC exposure.
Keywords: Immune complexes; Immunosenescence; Inflammaging; Monocytes activation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval This study was approved by the Ethics Committee of the Montpellier University Hospital (IRB No.: IRB-MTP_2022_04_202201101). All patients were included after providing written informed consent.
Figures




Similar articles
-
Elevated circulating monocytes and monocyte activation in COVID-19 convalescent individuals.Front Immunol. 2023 Apr 3;14:1151780. doi: 10.3389/fimmu.2023.1151780. eCollection 2023. Front Immunol. 2023. PMID: 37077911 Free PMC article.
-
Comprehensive single-cell chromatin and transcriptomic profiling of peripheral immune cells in nonsegmental vitiligo.Br J Dermatol. 2025 Jun 20;193(1):115-124. doi: 10.1093/bjd/ljaf041. Br J Dermatol. 2025. PMID: 39888372
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Zimmermann, P. & Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Child Dis.10.1136/archdischild-2020-320338 (2020). - PubMed
-
- Nikolich-Žugich, J. The twilight of immunity: Emerging concepts in aging of the immune system. Nat. Immunol.19, 10–19 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

