CTHRC1 Derived From Cancer-Associated Fibroblasts Promotes Pancreatic Cancer Progression and Metastasis via the LIF-STAT3 Pathway
- PMID: 40751418
- PMCID: PMC12317280
- DOI: 10.1002/cam4.71126
CTHRC1 Derived From Cancer-Associated Fibroblasts Promotes Pancreatic Cancer Progression and Metastasis via the LIF-STAT3 Pathway
Abstract
Background: Collagen triple helix repeat containing 1 (CTHRC1) is a secreted protein involved in tissue remodeling and fibrotic processes, which also suggests emerging roles in cancer. Studies have shown that it is mainly expressed in the outer membrane fibroblasts of injured arteries and in the neointimal smooth muscle cells, where it promotes cell migration and tissue damage repair. However, the regulatory role of CTHRC1 as a tumor microenvironment factor in pancreatic cancer is not well understood.
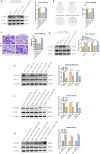
Methods: We employed multi-omics analysis combined with cellular and animal experiments to examine the association between CTHRC1 and the LIF/STAT3 pathway in pancreatic cancer clinical specimens. Using AsPC-1/PANC-1 and other cell lines, we conducted proliferation, migration, invasion, and signaling pathway studies, and elucidated the regulatory mechanism of CTHRC1 through genetic interventions and STAT3 inhibitors.
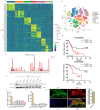
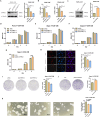
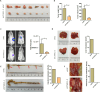
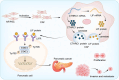
Results: In this study, we found that CTHRC1 is highly expressed in the cancer-associated fibroblasts (CAFs) of pancreatic cancer and is associated with poor prognosis in patients. Functionally, we observed that CTHRC1 in CAFs promotes the proliferation, migration, and invasion of pancreatic cancer cells both in vitro and in vivo. In mechanistic studies, RNA sequencing revealed that CTHRC1 promotes the proliferation and migration of pancreatic cancer cells through the LIF-mediated STAT3 axis.
Conclusion: These findings reveal the role of CTHRC1 in CAFs in pancreatic cancer, suggesting that it is an attractive therapeutic target and tumor marker. This study uncovers the biological mechanism of CTHRC1 in CAFs in pancreatic cancer, providing new strategies for the treatment of pancreatic cancer.
Keywords: CTHRC1; STAT3 signaling pathway; cancer‐associated fibroblast; pancreatic cancer; tumor microenvironment.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Senescent fibroblasts secrete CTHRC1 to promote cancer stemness in hepatocellular carcinoma.Cell Commun Signal. 2025 Aug 25;23(1):379. doi: 10.1186/s12964-025-02369-8. Cell Commun Signal. 2025. PMID: 40855439 Free PMC article.
-
β-Catenin mediated TAM phenotype promotes pancreatic cancer metastasis via the OSM/STAT3/LOXL2 axis.Neoplasia. 2025 Feb;60:101096. doi: 10.1016/j.neo.2024.101096. Epub 2024 Dec 30. Neoplasia. 2025. PMID: 39740539 Free PMC article.
-
WISP1 drives esophageal squamous cell carcinoma progression via modulation of cancer-associated fibroblasts and immune microenvironment.Front Immunol. 2025 Jul 23;16:1586790. doi: 10.3389/fimmu.2025.1586790. eCollection 2025. Front Immunol. 2025. PMID: 40771797 Free PMC article.
-
EV-mediated asymmetrical crosstalk between CAFs and cancer cells in the tumor microenvironment.Biochim Biophys Acta Rev Cancer. 2025 Sep;1880(4):189380. doi: 10.1016/j.bbcan.2025.189380. Epub 2025 Jul 3. Biochim Biophys Acta Rev Cancer. 2025. PMID: 40617309 Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Yu J., Yang X., He W., and Ye W., “Burden of Pancreatic Cancer Along With Attributable Risk Factors in Europe Between 1990 and 2019, and Projections Until 2039,” International Journal of Cancer 149, no. 5 (2021): 993–1001. - PubMed
-
- Huang J., Lok V., Ngai C. H., et al., “Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer,” Gastroenterology 160, no. 3 (2021): 744–754. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

