A Cluster Randomized Study to Explore Case Definitions, Clinical Course and Consequences of RSV in Community-Dwelling Adults Aged ≥ 50 Years
- PMID: 40751862
- PMCID: PMC12426295
- DOI: 10.1007/s40121-025-01205-3
A Cluster Randomized Study to Explore Case Definitions, Clinical Course and Consequences of RSV in Community-Dwelling Adults Aged ≥ 50 Years
Abstract
Introduction: In Europe, surveillance of respiratory syncytial virus (RSV) has been recently incorporated into existing influenza monitoring platforms that are based on influenza-like illness (ILI) or acute respiratory infection (ARI) case definitions. This study aims to compare RSV rates captured by ARI versus ILI case definitions and to describe the clinical and economic trajectories of RSV in older adults.
Methods: The study was conducted in Italy during the 2023/2024 and 2024/2025 seasons. Thirty-eight general practitioners were randomized 1:1 to enroll individuals ≥ 50 years presenting for care and meeting the European criteria for ARI or ILI, respectively. Alternative definitions were also explored. All subjects were tested for respiratory pathogens. RSV-positive individuals were followed for up to one month.
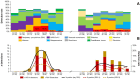
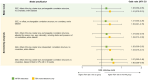
Results: Of 1431 patients (ARI: 741; ILI: 690) included, 5.2% tested positive for RSV. Odds of RSV in the ARI group (5.8%) was 26% higher than in the ILI group (4.6%) [odds ratio (OR) 1.26; 95% CI 0.60-2.65]. Exclusion of GPs with unexpectedly low enrollment rates increased the OR to 1.67 (95% CI 0.80-3.42). Conversely, adults in the ILI group showed higher rates of influenza A (OR 0.83; 95% CI 0.47-1.44) and SARS-CoV-2 (OR 0.57; 95% CI 0.34-0.95). A proposed alternative case definition, denoted as ARI with wheezing and/or productive cough and/or rhonchi and/or dyspnea was sensitive at 92.0% and specific at 30.8%. Among 75 RSV-positive outpatients, the case-complication, case-hospitalization and case-fatality rates were 30.7%, 2.7%, and 1.3%, respectively. The mean costs per RSV case were € 168.71 from the payer perspective and up to € 899.51 from the societal perspective.
Conclusions: Compared to a highly sensitive ARI definition, ILI-based surveillance likely underestimates the incidence of RSV. Further qualifiers can enhance specificity of the ARI case definition. The study confirms a significant burden of RSV in older adults.
Keywords: Acute respiratory infection (ARI); Burden of disease; Case definition; Complications; Cost of illness; Influenza-like illness (ILI); Older adults; RSV; Respiratory syncytial virus; Surveillance.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Alexander Domnich provided consultancies and/or received speaker fees from CSL Seqirus, GSK, Sanofi, and SD Biosensor. Francesco Lapi provided consultancies in protocol preparation for epidemiological studies and data analyses for CSL Seqirus, Moderna, AstraZeneca, Pfizer, and Viatris. Marta Vicentini, Anna Puggina, Alen Marijam, Maria João Fonseca and Elisa Turriani are employees of the GSK group of companies and may hold shares in the GSK group of companies as part of their employee remuneration. Andrea Orsi provided consultancies and/or received speaker fees from CSL Seqirus, Moderna, Novavax, and SD Biosensor. Donatella Panatto provided consultancies for Pfizer and CSL Seqirus and received grants for conducting observational studies from Sanofi, Pfizer, GSK, and Viatris. Giancarlo Icardi provided consultancies and/or received grants for conducting experimental and/or observational studies from GSK, Sanofi, MSD, CSL Seqirus, and Pfizer. Other authors declare no conflicts of interest. Ethical Approval: This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. The study protocol was approved by the Liguria Region Ethics Committee (#13354 of 18 September 2023). Written informed consent was obtained from all patients.
Figures
References
-
- Britton A, Roper LE, Kotton CN, Hutton DW, Fleming-Dutra KE, Godfrey M, et al. Use of respiratory syncytial virus vaccines in adults aged ≥60 years: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2024. MMWR Morb Mortal Wkly Rep. 2024;73(32):696–702. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

