Chimeric exosomes-derived immunomodulator restoring lymph nodes microenvironment for sensitizing TNBC immunotherapy
- PMID: 40753075
- PMCID: PMC12318024
- DOI: 10.1038/s41467-025-62543-x
Chimeric exosomes-derived immunomodulator restoring lymph nodes microenvironment for sensitizing TNBC immunotherapy
Abstract
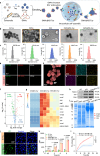
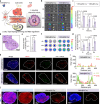
Immunotherapy is a breakthrough in the treatment of triple-negative breast cancer (TNBC), although it is only effective in a portion of patients. Our clinical studies find that pathological elevated level of reactive oxygen species (ROS) and lipid homeostasis imbalance are closely associated with dysfunction of dendritic cells (DCs) in the immunosuppressive lymph nodes (LNs) microenvironment of TNBC patients following immunotherapy, which greatly affect the immunotherapeutic efficacy. Building on this, we introduce a chimeric exosomes-derived immunomodulator involving the polysulfide bond-bridged mesoporous silica as both the ROS scavenger and responsive carrier nucleus, loading with the lipid modulator toyocamycin and being coated with chimeric exosomes comprising DCs-derived exosomes and Salmonella outer membrane vesicles. This multifaceted immunomodulator can significantly enhance LNs' homing through homologous targeting and chemokine-guided navigation, enabling ROS-responsive drug release, thereby restoring functions of DCs and LNs immuno-microenvironment. As expected, the immunomodulator significantly improves the responsiveness of TNBC to immunotherapy, exerting potent inhibition on both the primary tumor and metastases, while promoting a substantial increase in central memory T cells within LNs for sustained antitumor immunity. Our study provides a potent strategy for translational immunotherapy through optimizing the LNs microenvironment in TNBC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Leon-Ferre, R. A. & Goetz, M. P. Advances in systemic therapies for triple negative breast cancer. BMJ381, e071674 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

