B cell maturation antigen (BCMA) is dispensable for the survival of long-lived plasma cells
- PMID: 40753161
- PMCID: PMC12318048
- DOI: 10.1038/s41467-025-62530-2
B cell maturation antigen (BCMA) is dispensable for the survival of long-lived plasma cells
Abstract
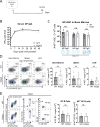
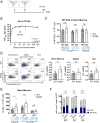
The survival of antibody-secreting plasma cells is essential for long-lasting humoral immunity. BCMA is proposed to promote APRIL-mediated survival signals. However, extensive shedding of murine BCMA raises doubts about its role as a signaling receptor. To unequivocally establish BCMA's function in plasma cell survival, we generate two BCMA-deficient mouse lines and examine antigen-specific plasma cells post-immunization. Contrary to previous reports, both BCMA-deficient mouse lines have comparable numbers of antigen-specific long-lived plasma cells following both protein and mRNA immunizations. Transcriptome analysis reveals no reduction in survival signaling upon BCMA deletion. Interestingly, BCMA-deficient mice show increased total plasma cell numbers in the bone marrow and mesenteric lymph nodes after boost immunizations. These results indicate that BCMA has no intrinsic role in maintaining long-lived plasma cells. Instead, we propose that BCMA's function is limited to acting as a soluble decoy receptor for APRIL, thereby fine-tuning the plasma cell population size by limiting survival factor availability. Our findings thus provide a strong argument against the APRIL-BCMA axis being a central mechanism for plasma cell longevity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Manz, R. A., Thiel, A. & Radbruch, A. Lifetime of plasma cells in the bone marrow. Nature388, 133–134 (1997). - PubMed
-
- Slifka, M. K., Antia, R., Whitmire, J. K. & Ahmed, R. Humoral immunity due to long-lived plasma cells. Immunity8, 363–372 (1998). - PubMed
-
- Robinson, M. J. et al. Intrinsically determined turnover underlies broad heterogeneity in plasma-cell lifespan. Immunity56, 1596–1612.e4 (2023). - PubMed
-
- Bossen, C. & Schneider, P. BAFF, APRIL and their receptors: structure, function and signaling. Semin. Immunol.18, 263–275 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

