Pericytes promote metastasis by regulating tumor local vascular tone and hemodynamics
- PMID: 40753165
- PMCID: PMC12318036
- DOI: 10.1038/s41467-025-62475-6
Pericytes promote metastasis by regulating tumor local vascular tone and hemodynamics
Abstract
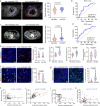
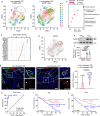
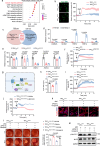
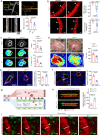
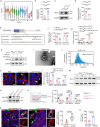
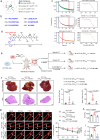
Hemodynamics are important for survival and extravasation of circulating tumor cells, whereas the effects of hemodynamics on tumor cell intravasation remain mostly unknown. Here, we show that colorectal cancer patients with liver metastasis are characterized by increased diameter and blood flow in the primary tumor compared with non-metastatic patients. A subpopulation of NKX2-3high tumor pericytes (TPCs) in the primary tumor is associated with hematogenous metastasis of patients. Mechanistically, long noncoding RNA NEAT1-enriched extracellular vesicles induce NKX2-3 upregulation in TPCs. NKX2-3 suppresses calcium influx in TPCs via PDE1C/cAMP/PKA signaling axis, inducing tumor vasodilation and increasing blood flux and vascular leakage. Genetic deletion of Nkx2-3 or pharmacological blocking the transcriptional activity of NKX2-3 in TPCs with designed peptide induce tumor local vasoconstriction and decrease blood flow to mitigate tumor metastasis. These findings reveal that TPCs-regulated vasodilation and hemodynamics facilitate tumor metastasis, and provide a potential prognostic marker and therapeutic strategy for tumor metastasis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
- 82273941/National Natural Science Foundation of China (National Science Foundation of China)
- 82404673/National Natural Science Foundation of China (National Science Foundation of China)
- 82003796/National Natural Science Foundation of China (National Science Foundation of China)
- 82204427/National Natural Science Foundation of China (National Science Foundation of China)
- 82304526/National Natural Science Foundation of China (National Science Foundation of China)
- 82204428/National Natural Science Foundation of China (National Science Foundation of China)
- 82473950/National Natural Science Foundation of China (National Science Foundation of China)
- 81973340/National Natural Science Foundation of China (National Science Foundation of China)
- 2024B1515020098/Natural Science Foundation of Guangdong Province (Guangdong Natural Science Foundation)
- 2024A1515013086/Natural Science Foundation of Guangdong Province (Guangdong Natural Science Foundation)
- 2019A1515110543/Natural Science Foundation of Guangdong Province (Guangdong Natural Science Foundation)
- 2020A1515010071/Natural Science Foundation of Guangdong Province (Guangdong Natural Science Foundation)
- 2021A1515110242/Natural Science Foundation of Guangdong Province (Guangdong Natural Science Foundation)
- 2023B1515120023/Natural Science Foundation of Guangdong Province (Guangdong Natural Science Foundation)
- 2025A04J5158/Guangzhou Science and Technology Program key projects
- SL2024A04J00374/Guangzhou Science and Technology Program key projects
- BX20230144/China Postdoctoral Science Foundation
- 2023M731323/China Postdoctoral Science Foundation
- 2022M711345/China Postdoctoral Science Foundation
- 2023M741390/China Postdoctoral Science Foundation
- 2022M721356/China Postdoctoral Science Foundation
- 2023M741391/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources
Medical

