hUC-MSCs and derived exosomes attenuate DEX-induced muscle atrophy through modulation of estrogen signaling pathway
- PMID: 40753405
- PMCID: PMC12318413
- DOI: 10.1186/s13287-025-04328-z
hUC-MSCs and derived exosomes attenuate DEX-induced muscle atrophy through modulation of estrogen signaling pathway
Abstract
Background: Sarcopenia, a multifactorial syndrome characterized by progressive loss of skeletal muscle mass and strength, combined with impaired physical function, is associated primarily with aging but also driven by chronic inflammation, immobility, and endocrine dysregulation. It leads to increased risks of frailty, falls, and loss of independence, posing a major public health challenge for aging populations. Although human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) and their derived exosomes (MSC-Exos) have demonstrated remarkable potential in regenerative medicine, their safety and efficacy in treating sarcopenia remain unclear. To address this issue, we conducted a preclinical study to systematically evaluate their therapeutic potential and safety.
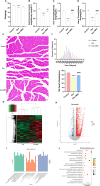
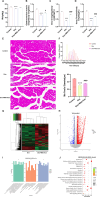
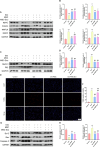
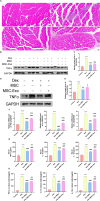
Methods: Male C57BL/6 J mice were treated with dexamethasone (20 mg/kg, i.p.) to induce muscle atrophy. Subsequently, bilateral intramuscular injection of hUC-MSCs (1 × 10⁶ cells/kg), exosomes (100 μg), or intraperitoneal injected of SNG162 (40 mg/kg) for two weeks. Gastrocnemius muscles were excised for histological analysis, TUNEL staining, Western blotting, RNA sequencing, and qPCR. Differentiated C2C12 myotubes were treated with 10 μM dexamethasone and co-cultured with hUC-MSCs or exosomes for 24 h. Samples were collected for qPCR, Western blot analyses and flow cytometry. EdU labeling was used to assess cell proliferation, MyHC and MDC immunofluorescence staining were employed to assess myotube morphology and autophagy levels, respectively. ELISA was used to quantify inflammatory cytokines and estrogen levels.
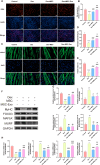
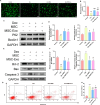
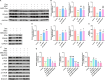
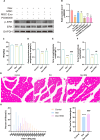
Results: hUC-MSCs, MSC-Exos and SNG162 improved grip strength and endurance in mice, increased the Gast muscle-to-body weight ratio without adversely affecting overall body weight, and enhanced muscle fiber cross-sectional area (CSA). Concurrently, they upregulated the expression of MyHC, Beclin-1, Bcl-2/Bax, ERα46, ERα36, ERβ and estradiol, while reducing key atrophy and inflammatory markers, including FOXO3, MAFbX, MURF1, TNF-α, IL-6, IL-1β, P62, and Caspase-3 in vitro and in vivo models. Furthermore, hUC-MSCs and MSC-Exos attenuated DEX-induced apoptosis in Gast muscles and C2C12 myotubes. Notably, MSC-Exos outperformed hUC-MSCs in promoting the proliferation and differentiation of C2C12 myotubes. Mechanistically, RNA sequencing and Western blot analysis identified the PI3K/AKT/mTOR and ERK1/2 signaling pathways as pivotal mediators of these effects.
Conclusions: This study underscores the potential of hUC-MSCs and their derived exosomes as a novel, safe, and effective therapeutic strategies for sarcopenia, offering promising avenues for clinical application.
Keywords: Apoptosis; Autophagy; Dexamethasone; Exosome; Human umbilical cord-derived mesenchymal stem cells; Muscle atrophy; Sarcopenia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: This study was performed at the Stem Cell Clinical Research Center of the First Affiliated Hospital of Dalian Medical University. This study was approved by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University (Project Title: Research on the Treatment of Sarcopenia Using Human Umbilical Cord Mesenchymal Stem Cells Combined with Phytomedicine; Approval No. YJ-GZR-SQ-2024–69; Approved on March 5, 2024). The ethical approval covered the collection of umbilical cord specimens, which were obtained from healthy fetuses delivered by cesarean section. Informed consent was provided by all pregnant women participants and their family members prior to specimen collection. All animal experiments were performed in strict accordance with the ethical guidelines established by the Animal Research Ethics Committee of Dalian Medical University (Project Title: Research on the Treatment of Sarcopenia with Human Umbilical Cord Mesenchymal Stem Cells Combined with Epimedium; Approval No. AEE24221; Approved on January 9, 2024). Consent for publication: All authors reviewed and endorsed the final version of the manuscript for submission and publication. Artificial intelligence (AI): The authors declare that no AI-generated content was used in this manuscript. Competing interests: The authors declare that they have no competing interests.
Figures
References
-
- Grima-Terrén M, Campanario S, Ramírez-Pardo I, Cisneros A, Hong X, Perdiguero E, et al. Muscle aging and sarcopenia: the pathology, etiology, and most promising therapeutic targets. Mol Aspects Med. 2024;100: 101319. - PubMed
-
- Sayer AA, Cooper R, Arai H, Cawthon PM, Ntsama Essomba M-J, Fielding RA, et al. Sarcopenia. Nat Rev Dis Primers. 2024;10:68. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

