Tailored interventions for inappropriate psychotropic drug use in nursing home residents with dementia: participatory action research in a special case of a stepped-wedge cluster randomized controlled trial
- PMID: 40753407
- PMCID: PMC12318394
- DOI: 10.1186/s12877-025-06206-y
Tailored interventions for inappropriate psychotropic drug use in nursing home residents with dementia: participatory action research in a special case of a stepped-wedge cluster randomized controlled trial
Abstract
Background: Psychotropic drugs are modestly effective and may cause adverse effects. Efforts to reduce inappropriateness and increase usage of psychosocial interventions often suffer from suboptimal implementation. The purpose of this study was to evaluate effectiveness of an innovative study using implementation promoting elements in nursing home residents with dementia and neuropsychiatric symptoms.
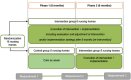
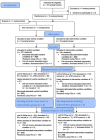
Methods: A multicenter cluster randomized controlled trial with a special case of a stepped-wedge design with two arms and one stap was designed. The intervention comprised participatory action research, tailored information provision and external coaching, leading to the implementation of tailored action and implementation plans. The primary outcome was inappropriateness of psychotropic drug use (Appropriate Psychotropic Drug Use in Dementia [APID] index) and the secondary outcome was percentage of psychotropic drug use at baseline, 8 months, and 16 months. Homes were allocated to start with usual care or the intervention. After 8 months, the control group crossed over to receive the intervention. The other homes continued the intervention to 16 months. Patients were eligible if they were diagnosed with dementia, had a life expectancy of at least 3 months, and resided in psychogeriatric units.
Results: An adjusted multilevel model revealed no effect on the APID index sum score at 8 months (0.564; 95% confidence interval [CI], -2.449-3.577; p = 0.71) or 16 months (2.165; 95% CI, -1.113-5.443; p = 0.20). An adjusted generalized estimation equation (GEE) model showed an effect at 16 months for percentage of use (OR 0.654; 95% CI, 0.481-0.889; p = 0.007). Adjusted GEE models showed an effect especially at 16 months for anxiolytics (OR 0.573; 95% CI, 0.382-0.859; p = 0.007) and antidepressants (OR 0.678; 95% CI, 0.475-0.968; p = 0.033).
Conclusions: No reduction of inappropriateness was found although overall usage was reduced. Professionals focused on implementing alternatives to compensate for usage, rather than prescribing quality. Future studies may focus on changing physicians' prescribing behaviors in combination with multicomponent and multidisciplinary psychosocial alternatives.
Trial registration: Netherlands Trial Registry (NTR5872) on 27/05/2016, https://onderzoekmetmensen.nl/nl/node/26060/pdf .
Keywords: Complex interventions; Dementia; Neuropsychiatric symptoms; Nursing homes; Psychotropic drugs.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Legal representatives of eligible residents were approached in writing to inform them about the study. Written informed consent was obtained for all participants. The Medical Ethics Review Board of the University Medical Centre Groningen has assessed the study and concluded that this non-invasive study did not constitute clinical research with human subjects, as meant by the Dutch Medical Research Involving Human Subjects Act (METC decision: METc 2016.094). The study was conducted in accordance with the declaration of Helsinki and the General Data Protection Regulation. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- World Health Organization. Dementia [Internet]. 2023 [cited 2023 Apr 4]. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia.
-
- Zuidema SU, Derksen E, Verhey FRJ, Koopmans RTCM. Prevalence of neuropsychiatric symptoms in a large sample of Dutch nursing home patients with dementia. Int J Geriatr Psychiatry. 2007;22(7):632–8. - PubMed
-
- Selbæk G, Engedal K, Bergh S. The prevalence and course of neuropsychiatric symptoms in nursing home patients with dementia: A systematic review. J Am Med Dir Assoc. 2013;14(3):161–9. - PubMed
-
- Taipale H, Koponen M, Tanskanen A, Lavikainen P, Tolppanen AM, Sund R, et al. Use of benzodiazepines and related drugs is associated with a risk of stroke among persons with alzheimer’s disease. Int Clin Psychopharmacol. 2017;32(3):135–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical