Trauma resuscitation with Low-Titer Group O Whole Blood Or Products: study protocol for a randomized clinical trial (the TROOP trial)
- PMID: 40753420
- PMCID: PMC12318426
- DOI: 10.1186/s13063-025-08971-y
Trauma resuscitation with Low-Titer Group O Whole Blood Or Products: study protocol for a randomized clinical trial (the TROOP trial)
Abstract
Background: Hemorrhage is the most common cause of potentially preventable death after injury. Balanced transfusion with red blood cells, plasma, and platelets (component therapy, CT) has been shown to reduce mortality, and is the standard of care. Low-Titer Group O Whole Blood (LTOWB) is an attractive alternative to CT, but existing evidence comprises observational studies, and a small single center pilot randomized controlled trial, which evaluated a type of whole blood that is no longer in use. The aim of the "Trauma Resuscitation with Low-Titer Group O Whole Blood Or Products" (TROOP) trial is to compare the effectiveness and safety of LTOWB and CT in critically injured patients predicted to require a large volume transfusion.
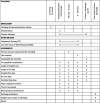
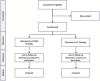
Methods: This is a pragmatic, multicenter, Bayesian, sequential non-inferiority/superiority, randomized clinical trial, performed within 15 level I trauma centers in the United States. We aim to randomize 1,100 injured patients to resuscitation with either CT or LTOWB. The primary outcome is 6-h mortality. Secondary outcomes include 24-h and 30-day or hospital mortality (whichever is earlier); prespecified complications; adjudicated cause of death; time to death; length of stay (ICU and hospital); and hospital-, ventilator- and ICU-free days; the incidence of major surgical procedures; time to hemostasis in those undergoing procedures with a hemostatic component; number and type of blood products used until hemostasis is achieved (and randomized products are discontinued), as well as after hemostasis has been achieved, to 24 h post-admission; discharge destination and functional status and quality of life at hospital discharge or 30 days, as measured by Glasgow Coma Scale (GCS) and EuroQol (EQ-5D) quality of life measurement.
Discussion: This large multicenter clinical trial will contribute high-level evidence on the effectiveness of Low-Titer Group O Whole Blood in the in-hospital management of trauma patients predicted to require a large volume transfusion. Trial registration National Clinical Trial Identified Number: NCT05638581.
Clinical trial registry: https://clinicaltrials.gov/study/NCT05638581 First submitted 2022-11-08.
Keywords: Component therapy; Injury; Low-Titer Group O Whole Blood; Resuscitation; Trauma.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate {24}: The trial has been approved by Advarra. Consent for publication {32}: Sample consent forms are included in the appendix. No identifying images or other personal or clinical details of participants are presented here or will be presented in reports of the trial results. Competing interests {28}: JOJ has received grants from NIH, NIHR, DoD, and MTEC and is a consultant for CSL Behring, Cellphire, Infrascan, and Octapharm. JBH is on the board of directors of CCJ Medical Devices, QinFlow, Hemostatics, and Zibrio; receives research grant support from the DoD, DARPA, NIH, and CSL focused on hemorrhage control and resuscitation. He also consults with WFIRM, Infrascan, Geneva, Aspen Medical and is the co-inventor of the Junctional Emergency Tourniquet Tool and thus receives royalties from UT Health.
Figures

References
-
- Berwick D, Downey A, Cornett E. A National Trauma Care System: Integrating Military and Civilian Trauma Systems to Achieve Zero Preventable Deaths After Injury. Washington, D.C.: National Academies of Sciences, Engineering, and Medicine; 2016. - PubMed
-
- Tien H, Spencer F, Tremblay L, Rizoli S, Brenneman F. Preventable Deaths From Hemorrhage at a Level I Canadian Trauma Center. J Trauma Inj Infect Crit Care. 2007;62(1):142–6. - PubMed
-
- Davis JS, Satahoo SS, Butler FK, Dermer H, Naranjo D, Julien K, et al. An analysis of prehospital deaths: Who can we save? J Trauma Acute Care Surg. 2014;77(2):213. - PubMed
-
- Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82(3):605–17. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

