A Phase IIa, Single-Blind, Placebo-Controlled, Parallel-Group Study to Assess Safety, Tolerability, and Pharmacokinetics/Pharmacodynamics of Brensocatib in Adults with Cystic Fibrosis
- PMID: 40753522
- PMCID: PMC12479633
- DOI: 10.1007/s40262-025-01550-z
A Phase IIa, Single-Blind, Placebo-Controlled, Parallel-Group Study to Assess Safety, Tolerability, and Pharmacokinetics/Pharmacodynamics of Brensocatib in Adults with Cystic Fibrosis
Abstract
Background and objectives: Brensocatib, an oral, competitive, and reversible inhibitor of dipeptidyl peptidase 1 (DPP1), reduces exacerbations and lung function decline in non-cystic fibrosis bronchiectasis (NCFBE). This study aimed to evaluate the pharmacokinetics (PK), pharmacodynamics (PD), safety, and tolerability of brensocatib in adults with cystic fibrosis (CF), comparing these findings with data from previous trials in healthy adults and in those with NCFBE to inform dose selection for future clinical trials.
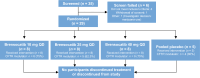
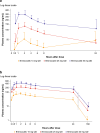
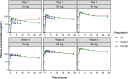
Methods: A phase IIa, single-blind, randomized, placebo-controlled trial was conducted to assess the PK, PD, safety, and tolerability of brensocatib in adults with CF. Participants were randomly assigned to receive once-daily brensocatib (10 mg, 25 mg, or 40 mg) or placebo for 28 days. The study planned enrollment of up to 34 adults, stratified on the basis of their CF transmembrane conductance regulator (CFTR) modulator use, to evaluate the PK profile of brensocatib and its safety compared with placebo. Primary PK parameters, including maximum plasma concentration (Cmax), time to maximum concentration (Tmax), area under the concentration-time curve from 0 to 24 h (AUC0-24), and half-life (t1/2), were determined on day 1 and day 28. Dose-dependency of brensocatib exposure was analyzed, and safety and tolerability were assessed through treatment-emergent adverse events. Data from participants were compared with previous data from healthy adults and from those with NCFBE.
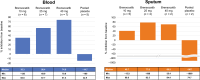
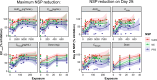
Results: A total of 29 participants were randomized to treatment, with 21 stratified to the CFTR modulator group. Baseline characteristics were similar among cohorts. Mean age was 37.9 (standard deviation (SD) 14.6) years, and most participants exhibited mild-to-moderate lung disease. PK analysis showed dose-dependent and predictable brensocatib exposure, with comparable profiles between participants with and without use of CFTR modulators. In addition, PK profiles in participants were comparable to those of healthy adults and of those with NCFBE. Pharmacodynamic analysis revealed dose-dependent reduction in neutrophil serine protease (NSP) activity, reaching saturation around the 25-mg dose, particularly in blood. Brensocatib at all doses was well tolerated with no new identified safety signals.
Conclusions: Brensocatib demonstrated consistent PK profiles independent of CFTR therapy and comparable to those of healthy and NCFBE adults. Brensocatib reduced blood and sputum NSP levels. The safety profile was comparable to previous studies, with no new safety concerns identified, supporting the use of similar dosing for adults with CF as for other populations. These findings advocate for further investigation of brensocatib in CF.
Clinical trial registration: NCT05090904.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Funding: This study was funded by Insmed Incorporated (Bridgewater, NJ, USA). Conflicts of Interest: Michael Konstan reports consulting fees from Insmed Incorporated. James Tolle and Emily DiMango have nothing to disclose. Patrick Flume reports consulting fees and research support from Insmed Incorporated. Helen Usansky, Ariel Teper, Christina Ramirez, Jimmy Flarakos, Jessica Basso, Sherry Li, and Marcela Vergara are employees and shareholders in Insmed Incorporated. Ethics Approval: This study was conducted in accordance with the protocol and consensus ethical principles derived from international guidelines, including the Declaration of Helsinki, Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable International Council for Harmonisation Good Clinical Practices Guidelines, and other applicable laws and regulations. Consent to Participate: Participants and/or their legally authorized representative were informed that their participation was voluntary. Participants or their legally authorized representative were required to sign a statement of informed consent that met the requirements of ICH E6 (R2) guideline for good clinical practice (GCP) and any additional elements required by local regulations. Consent for Publication: Not applicable. Availability of Data and Material: The datasets generated and/or analyzed during the current study are not publicly available owing to patient privacy. Authors’ Contributions: Helen Usansky, Ariel Teper, and Marcela Vergara contributed to the study conception and design. All authors contributed to data collection, analysis, and interpretation. All authors critically reviewed all manuscript drafts, approved the submitted version of the manuscript, and made the decision to submit the manuscript for publication.
Figures

References
-
- Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol. 1997;24(2):137–42. https://onlinelibrary.wiley.com/doi/10.1002/(SICI)1099-0496(199708)24:2%... - DOI - PubMed
-
- Berger M. Inflammatory mediators in cystic fibrosis lung disease. Allergy Asthma Proc. 2002;23(1):19-25. https://www.ingentaconnect.com/content/ocean/aap/2002/00000023/00000001/... - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

