Clinical course of patients with bloodstream infections enrolled in the BALANCE clinical trial
- PMID: 40754718
- PMCID: PMC12494135
- DOI: 10.1093/jac/dkaf294
Clinical course of patients with bloodstream infections enrolled in the BALANCE clinical trial
Abstract
Objectives: There is a lack of data describing the longitudinal clinical trajectories of vital signs and laboratory tests in patients with bloodstream infection (BSI). The BALANCE trial, which randomly assigned patients with BSI to receive 7 or 14 days of antibiotic treatment, provided rich daily data to describe these trajectories.
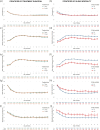
Methods: As part of the BALANCE trial, we collected several daily parameters (temperature, heart rate, mean arterial pressure, systolic blood pressure, respiratory rate, WBC count, C-reactive protein, platelet count and SOFA score) until Day 14 of illness, discharge or death. In this post hoc descriptive sub-study, we described trajectories of these parameters, stratified by treatment group allocation and by the primary outcome of 90-day all-cause mortality.
Results: Among 3608 patients included, median age was 70 years and 46.7% were female. At enrolment, 55.0% were admitted in the ICU and 21.2% required mechanical ventilation. Longitudinal trajectories of vital signs, laboratory tests and SOFA scores were almost identical comparing the two treatment groups, including from Day 7 after treatment divergence. These trajectories were markedly different when comparing survivors (3034 patients; 84.7%) and non-survivors (547 patients; 15.3%), with non-survivors demonstrating a slower recovery course throughout the 14-day period.
Conclusions: Among hospitalized patients with BSI, recovery trajectories were similar in patients assigned to 7- versus 14-day antibiotic treatment durations but were different comparing survivors versus non-survivors. These data could be used to inform daily clinical management, formulate predictive risk scores or clinical decision rules, and guide future research into individualized therapeutic strategies.
© The Author(s) 2025. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

