Evaluating the Combined Effect of a Choline Kinase Inhibitor and Temozolomide Therapy in a Mouse Model of Glioblastoma Using 1H MR Spectroscopy and IVIM-DWI
- PMID: 40755153
- PMCID: PMC12319478
- DOI: 10.1002/nbm.70113
Evaluating the Combined Effect of a Choline Kinase Inhibitor and Temozolomide Therapy in a Mouse Model of Glioblastoma Using 1H MR Spectroscopy and IVIM-DWI
Abstract
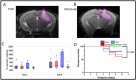
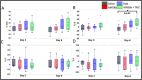
This study evaluated the therapeutic efficacy of combining a choline kinase alpha (ChoKα) inhibitor, MN58b, and temozolomide (TMZ) in a syngeneic GL261 glioblastoma (GBM) mouse model. It used MR spectroscopy (MRS) and intravoxel incoherent motion diffusion-weighted imaging (IVIM-DWI) to assess metabolic and microstructural changes within the tumor. Fifty-two C57BL/6 mice had GL261 cells implanted intracranially and were divided into four groups: saline control, MN58b, TMZ, and MN58b + TMZ (n = 16, 14, 11, and 11, respectively). Treatments were administered for 5 days, starting 10 days post-implantation. MRI scans (T2-weighted, MRS, IVIM-DWI) were performed at baseline, during treatment (Day 3), and post-treatment (Day 6). The histological analysis evaluated the tumor mitotic index and caspase-3 expression. Combination therapy with MN58b + TMZ significantly reduced tCho/NAA, Lip + Lac/tCr, and mI/tCr, suggesting decreased phosphocholine synthesis and tumor proliferation. IVIM-DWI showed a significant increase in diffusion coefficient (D) values, indicating reduced cell density. Metabolic changes detected by 1H MRS were observable as early as Day 3 post-treatment initiation, preceding microstructural alterations detected by IVIM-DWI at Day 6. This suggests that MRS biomarkers may serve as early indicators of treatment response, facilitating timely therapeutic decisions. Histology confirmed a significantly lower mitotic index relative to control tumors in the combination treatment group. Significantly prolonged survival with combination therapy was noted relative to other groups. However, the tumor volumes were not significantly different between groups. Combination therapy targeting ChoKα and cellular proliferation with MN58b and TMZ outperformed individual treatments for GBM, warranting further exploration. The integration of MN58b with the current standard of care (TMZ + RT) might further enhance the therapeutic outcomes. MRS and IVIM-DWI demonstrate potential utility as non-invasive imaging markers for treatment monitoring.
Keywords: GBM; IVIM; MR spectroscopy; cancer; glioblastoma; microstructure; treatment response.
© 2025 The Author(s). NMR in Biomedicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Fèvre L., Lhermitte C., Ahle B., et al., “Pseudoprogression Versus True Progression in Glioblastoma Patients: A Multiapproach Literature Review: Part 1—Molecular, Morphological and Clinical Features,” Critical Reviews in Oncology/Hematology 157 (2021): 103188, 10.1016/j.critrevonc.2020.103188. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

