When Is Postpartum Haemorrhage Treatment Initiated? A Nested Observational Study Within the E-MOTIVE Trial
- PMID: 40755244
- PMCID: PMC12411655
- DOI: 10.1111/1471-0528.18293
When Is Postpartum Haemorrhage Treatment Initiated? A Nested Observational Study Within the E-MOTIVE Trial
Abstract
Objective: To compare the frequency and timing of postpartum haemorrhage (PPH) treatment initiation between hospitals implementing the MOTIVE treatment bundle (which consisted of uterine Massage, Oxytocic drugs, Tranexamic acid, IntraVenous fluids and Examination) and those following usual care.
Design: Nested prospective observational study.
Setting: Hospitals in Nigeria, Kenya, Tanzania and South Africa participating in the E-MOTIVE trial.
Population or sample: Healthcare workers treating PPH.
Methods: Between June and December 2022, we observed healthcare workers for 1-2 weeks in 39 E-MOTIVE and 39 usual care hospitals across Nigeria, Kenya, Tanzania, and South Africa managing vaginal birth and treating PPH. We descriptively compared the frequency and timing from PPH detection to treatment initiation of individual treatments and the MOTIVE bundle, between E-MOTIVE care and usual care.
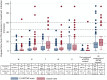
Results: Among 2578 observations in E-MOTIVE care hospitals, 295 (11%) PPHs were treated, and among 2834 observations in usual care hospitals, 219 (8%) PPHs were treated. In E-MOTIVE care hospitals, 97% (286/295) of women with PPH received the MOTIVE bundle, compared to 36% (79/219) in usual care. Median initiation times for the first component were similar (0 vs. 1 min), but E-MOTIVE care hospitals achieved faster initiation of all components (13 min, IQR 6-18) compared to usual care (18 min, IQR 10-25). In total, 79% (233/295) of women in E-MOTIVE care had all components initiated within 20 min, compared to 22% (48/219) in usual care.
Conclusions: Timely and comprehensive management of PPH using the MOTIVE bundle, particularly initiating all components within 15-20 min, was commonly observed in the E-MOTIVE care hospitals. Scaling up E-MOTIVE care should emphasise timely bundle initiation to strengthen PPH treatment and improve maternal health outcomes in low-and-middle-income countries.
Keywords: MOTIVE bundle; PPH; PPH treatment; PPH treatment bundle; postpartum haemorrhage; sub‐Saharan Africa.
© 2025 The Author(s). BJOG: An International Journal of Obstetrics and Gynaecology published by John Wiley & Sons Ltd.
Conflict of interest statement
Justus Hofmeyr has conceived a re‐usable device for postpartum blood loss monitoring, the Maternawell Tray, which is marketed by Maternova, a global women's health solutions company that holds the intellectual property. JH benefited from consulting fees in the past as inventor but receives no current income nor prospect of future income from this project. The remaining authors declare that they have no competing interests.
Figures
References
-
- World Health Organisation , A Roadmap to Combat Postpartum Haemorrhage Between 2023 and 2030 (World Health Organisation, 2023).
-
- World Health Organisation , Trends in Maternal Mortality 2000 to 2020: Estimated by WHO, UNICEF, UNFPA, World Bank Group and UNDESA /Population Division (World Health Organization, 2023).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

