Unraveling the Mn2+ substitution effect on the anisotropy control and magnetic hyperthermia of MnxFe3- xO4 nanoparticles
- PMID: 40755348
- PMCID: PMC12319668
- DOI: 10.1039/d5nh00254k
Unraveling the Mn2+ substitution effect on the anisotropy control and magnetic hyperthermia of MnxFe3- xO4 nanoparticles
Abstract
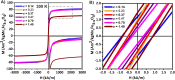
Composition is a key parameter to effectively tune the magnetic anisotropy of magnetic nanoparticles, which in turn can modulate their structural-magnetic properties and final applications. The Mn2+ content of manganese ferrite nanoparticles (MnxFe3-xO4) deeply impacts their structure, anisotropy, magnetism, and their heating capacity. However, a direct correlation between Mn2+ content, magnetic properties and heating efficiency is not yet clear. Herein, we report the synthesis of a wide range of MnxFe3-xO4 with x = 0.14 to 1.40, with similar polyhedral morphologies and sizes (13 to 15 nm). By varying the Mn2+ content (in the range of x = 0.0 up to 0.70), we successfully tuned the effective anisotropy while maintaining saturation magnetization nearly constant. Highest Mn2+ levels (x = 1.40) lead to structural changes and strain defects reflected in their poor saturation magnetization. Mn2+ substitution is not uniform, instead promotes a compositional gradient across the MNPs, with the surface layers having a higher concentration of Mn2+ than the core. The Mn2+-rich surface likely exhibits superparamagnetic (SPM) relaxation, while the core remains predominantly ferrimagnetic (FiM). Water transference results in cation leaching, promoting vacancies and changes in the local ferrite structure but with a minor impact on the magnetic properties compared with initial MNPs. We obtained the optimal Mn2+ content that maximizes anisotropy toward improved specific loss power (SLP) values. The Néel relaxation mechanism is warranted regarding variable composition when sizes and shapes are maintained. Our detailed analysis provides a better understanding of the effect of Mn2+ substitution on the heating efficiency through anisotropy modulation and straightforward guidance on optimizing MNP design for magnetic hyperthermia.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Gavilán H. Avugadda S. K. Fernández-Cabada T. Soni N. Cassani M. Mai B. T. Chantrell R. Pellegrino T. Chem. Soc. Rev. 2021;50:11614–11667. - PubMed
-
- Yang L. Ma L. Xin J. Li A. Sun C. Wei R. Ren B. W. Chen Z. Lin H. Gao J. Chem. Mater. 2017;29:3038–3047.
-
- Del Sol Fernández S. Odio O. F. Crespo P. M. Pérez E. O. Salas G. Gutiérrez L. del M. Morales P. Reguera E. J. Phys. Chem. C. 2022;126:10110–10128.
-
- Li D. Yun H. Diroll B. T. Doan-Nguyen V. V. T. Kikkawa J. M. Murray C. B. Chem. Mater. 2016;28:480–489.
LinkOut - more resources
Full Text Sources

