Endothelial-Derived CCL7 Promotes Macrophage Polarization and Aggravates Septic Acute Lung Injury via CCR1-Mediated STAT1 Succinylation
- PMID: 40755420
- PMCID: PMC12520477
- DOI: 10.1002/advs.202506209
Endothelial-Derived CCL7 Promotes Macrophage Polarization and Aggravates Septic Acute Lung Injury via CCR1-Mediated STAT1 Succinylation
Abstract
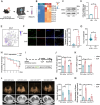
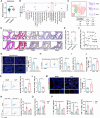
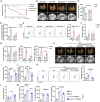
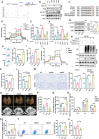
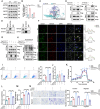
Acute lung injury (ALI) is a significant complication of sepsis, wherein the interaction between pulmonary vascular endothelial cells and immune cells plays a pivotal role in the pathogenesis. In this study, it is demonstrated that secretion of chemokine C-C motif ligand 7 (CCL7) by endothelial cells (ECs) induces metabolic reprogramming and M1 polarization of C-C motif chemokine receptor 1-positive (CCR1⁺) macrophages. It is noteworthy that mice with specific inhibition of endothelial-derived CCL7 exhibit reduced severity of septic ALI, underscoring the critical role of CCL7 in the progression of sepsis. Mechanistically, activation of the CCL7-CCR1 axis enhances STAT1 succinylation through upregulation of KAT2A expression, leading to increased STAT1 binding to the promoter of glycolytic genes in macrophages. This epigenetic regulation modulates metabolic reprogramming and M1 polarization of macrophages, thereby driving inflammatory cascades in septic ALI. Furthermore, in sepsis models, Ccr1-knockout (Ccr1-KO) mice demonstrate attenuated lung inflammation and decreased mortality, highlighting the therapeutic potential of targeting the CCL7-CCR1 axis for the treatment of septic ALI. Collectively, findings provide novel insights into the metabolic reprogramming of macrophages and identify the CCL7-CCR1 axis as a promising therapeutic target for septic ALI.
Keywords: CCL7; glycolysis; macrophage; sepsis; succinylation.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Parotto M., Gyongyosi M., Howe K., Myatra S. N., Ranzani O., Shankar‐Hari M., Herridge M. S., Lancet Respir Med 2023, 11, 739. - PubMed
-
- Meyer N. J., Prescott H. C., N. Engl. J. Med. 2024, 391, 2133. - PubMed
-
- Zhang J., Wang T., Wang Y., Li Y., Wang L., Wang J., Miao Y., Xu F., Yao Y., Cell Rep. 2025, 44, 115571. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
