Albiflorin improves diabetic retinopathy by mitigating oxidative stress and inflammation via the TLR-4/NF-kB signaling pathway
- PMID: 40755526
- PMCID: PMC12315536
- DOI: 10.1093/toxres/tfaf105
Albiflorin improves diabetic retinopathy by mitigating oxidative stress and inflammation via the TLR-4/NF-kB signaling pathway
Abstract
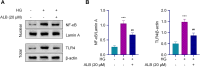
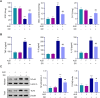
This study was to investigate the effects of Albiflorin (ALB) on oxidative stress and inflammation in diabetic retinopathy (DR) and explore its potential mechanism involving the Toll-like receptor 4 (TLR4)/nuclear factor kappa B (NF-κB) signaling pathway. Human retinal microvascular endothelial cells (HRMECs) were treated with high glucose (HG) and ALB. Cell viability was assessed by MTT assay. Oxidative stress markers and inflammatory cytokines were measured by ELISA. TLR4/NF-κB pathway proteins were analyzed by Western blot. A streptozotocin (STZ)-induced diabetic rat model was established to examine retinal histological changes. Serum metabolic parameters, oxidative stress markers, and inflammatory cytokines were evaluated in the DR model and ALB intervention groups. Results showed that ALB improved HRMEC viability under HG induction and reduced oxidative stress and inflammation. ALB inhibited the TLR4/NF-κB pathway in HG-induced HRMECs. Overexpression of TLR4 partially reversed the protective effects of ALB. In diabetic rats, ALB ameliorated metabolic disorders, improved retinal histological structure, and reduced oxidative stress and inflammation. ALB also suppressed the TLR4/NF-κB signaling pathway in vivo. In conclusion, ALB improves DR by resolving oxidative stress and inflammation through inhibiting the TLR4/NF-κB signaling pathway. These findings suggest ALB as a potential therapeutic agent for DR.
Keywords: Albiflorin; TLR-4/NF-κB signaling; diabetic retinopathy; inflammation; oxidative stress.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
The authors declared that there was no conflict of interest associated with the manuscript.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

