Sodium 2-Mercaptoethanesulfonate (MESNA), Ifosfamide, Mitoxantrone, and Etoposide (MINE) in Transplant-Ineligible Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Is the Old Regimen Still Gold?
- PMID: 40755587
- PMCID: PMC12314218
- DOI: 10.7759/cureus.87128
Sodium 2-Mercaptoethanesulfonate (MESNA), Ifosfamide, Mitoxantrone, and Etoposide (MINE) in Transplant-Ineligible Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Is the Old Regimen Still Gold?
Abstract
Introduction: For decades, the rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisolone (R-CHOP) regimen has been the standard treatment for aggressive B-cell non-Hodgkin lymphoma (NHL), such as diffuse large B-cell lymphoma (DLBCL). However, patients with relapsed or refractory (R/R) disease continue to face a poor prognosis. Those eligible for autologous hematopoietic stem cell transplantation (ASCT) are usually rescued with a platinum-containing regimen. Conversely, milder regimens are preferred for ineligible patients, such as gemcitabine and oxaliplatin (GemOx). At our institution, the standard second-line treatment for patients over 65 years or with comorbidities that make them unsuitable for ASCT is a non-platinum-based regimen composed of sodium 2-mercaptoethanesulfonate (MESNA), ifosfamide, mitoxantrone, and etoposide (MINE). Although newer targeted and immune-based therapies are emerging, there remains a lack of prospective studies on optimal treatment choices for this group of patients, particularly regarding non-platinum-based regimens.
Aim: The study aimed to evaluate in a real-world setting the efficacy and safety profile of MINE, with or without rituximab, in patients with R/R DLBCL.
Methods: This was a retrospective, single-center study conducted from April 2007 to August 2024. It included patients who underwent at least one cycle of MINE. Data were collected from patients' electronic records. The primary endpoints were overall survival (OS), progression-free survival (PFS), and duration of response (DoR). The secondary endpoints included complete response (CR) and overall response rates (ORR), as well as toxicity-related surrogates, such as the total number of required RBC units or platelet concentrates (PC), total number of febrile neutropenia (FN) episodes, ICU admissions, and treatment-emergent adverse events (TEAEs).
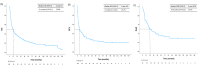
Results: A total of 167 patients were included, with a median follow-up time of 10 months (IQR: 4-40), a female-to-male ratio of 1.26, and 127/167 (76.0%) patients over the age of 65 (median age: 70). About 47/167 (28.1%) cases resulted from the transformation of indolent NHL. Disease was localized in 94/151 (62.3%), and 52/166 (31.3%) were refractory to the previous treatment. The protocol was used as second-line therapy in 121/167 (72.4%), with rituximab added in 86/167 (51.5%). Median OS, PFS, and DoR were 11, seven, and 24 months, respectively. In univariate analysis, PFS and OS were significantly higher in patients receiving rituximab with MINE and lower in those who presented with bulky masses, advanced-stage disease, and refractoriness to the prior line. CR was 45.3% and ORR was 63.5%. Myelotoxicity was the primary complication, with 38/124 (30.6%) patients developing FN, and six (4.8%) requiring ICU admission. This was followed by cardiotoxicity in 18/124 (14.5%). Treatment was discontinued in 17/152 (11.2%) patients, and 11/152 (7.2%) died due to toxicity.
Conclusions: This retrospective study demonstrates that the MINE protocol offers favorable outcomes and an acceptable safety profile, with myelotoxicity as the most significant adverse effect. Although inclusion criteria were not strictly limited to ineligible patients, they constituted the majority of this cohort. In conclusion, while additional prospective studies are needed, these findings reinforce MINE as a still viable and cost-efficient alternative, particularly for R/R DLBCL patients who are not eligible for ASCT.
Keywords: autologous hematopoietic stem cell transplantation; diffuse large b-cell lymphoma (dlbcl); etoposide; ifosfamide; mitoxantrone; non-hodgkin lymphoma; systemic chemotherapy.
Copyright © 2025, Maia Moço et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Comissão de Ética para a Saúde (ESC) - Instituto Português de Oncologia do Porto Francisco Gentil issued approval CES.020_25. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Diffuse large B-cell lymphoma. Li S, Young KH, Medeiros LJ. Pathology. 2018;50:74–87. - PubMed
-
- Navigating the evolving treatment landscape of diffuse large B-cell lymphoma. Nastoupil LJ, Bartlett NL. J Clin Oncol. 2023;41:903–913. - PubMed
-
- Results of a salvage treatment program for relapsing lymphoma: MINE consolidated with ESHAP. Rodriguez MA, Cabanillas FC, Velasquez W, Hagemeister FB, McLaughlin P, Swan F, Romaguera JE. J Clin Oncol. 1995;13:1734–1741. - PubMed
-
- Phase II study of a high-dose ifosfamide-based chemotherapy regimen with growth factor rescue in recurrent aggressive NHL. High response rates and limited toxicity, but limited impact on long-term survival. van Besien K, Rodriguez A, Tomany S, et al. Bone Marrow Transplant. 2001;27:397–404. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous