Development and validation of a machine learning-based nomogram for preoperative prediction of laparoscopic surgical difficulty in gallstone patients
- PMID: 40755730
- PMCID: PMC12314664
- DOI: 10.21037/tgh-24-124
Development and validation of a machine learning-based nomogram for preoperative prediction of laparoscopic surgical difficulty in gallstone patients
Abstract
Background: Preoperative prediction of laparoscopic surgical difficulty in gallstone patients is crucial for improving surgical outcomes. This study aimed to develop and validate a nomogram based on advanced machine learning algorithms, incorporating key clinical and systemic inflammatory response indicators, such as the C-reactive protein to albumin ratio (CAR).
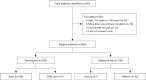
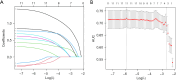
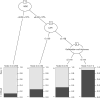
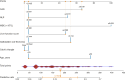
Methods: A retrospective analysis was conducted on 362 eligible patients who underwent laparoscopic cholecystectomy (LC) for gallstones between 2013 and 2019. A total of 420 patients were initially identified, with 58 excluded based on predefined criteria such as age and incomplete records. The remaining patients were divided into a training set (n=253) and a validation set (n=109). The development of the nomogram involved multiple analytical techniques, including machine learning methods such as least absolute shrinkage and selection operator (LASSO) regression, decision tree analysis, and support vector machine (SVM) models, along with traditional statistical methods like univariate and multivariate logistic regression. Significant predictors, including CAR, white blood cell count (WBC), and gallbladder wall thickness, were integrated into the final predictive model. Model performance was evaluated using receiver operating characteristic (ROC) curve analysis and calibration plots.
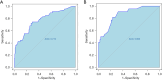
Results: The machine learning-based model demonstrated strong predictive capability, with an area under the curve (AUC) of 0.774 in the training set and 0.863 in the validation set. Calibration plots showed good agreement between predicted and actual outcomes, with mean absolute errors of 0.035 and 0.05 for the training and validation sets, respectively.
Conclusions: This study demonstrates the utility of applying machine learning algorithms to develop a robust nomogram for preoperative prediction of laparoscopic surgical difficulty. By integrating key clinical variables and systemic inflammatory markers, the model provides an effective tool for improving surgical planning and enhancing patient outcomes.
Keywords: Laparoscopic surgery; machine learning; nomogram; preoperative prediction; systemic inflammatory response.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-124/coif). The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Clinical diagnostic and prognostic value of homocysteine combined with hemoglobin [f (Hcy-Hb)] in cardio-renal syndrome caused by primary acute myocardial infarction.J Transl Med. 2025 Jul 23;23(1):813. doi: 10.1186/s12967-025-06512-4. J Transl Med. 2025. PMID: 40702542 Free PMC article.
-
Prediction of additional hospital days in patients undergoing cervical spine surgery with machine learning methods.Comput Assist Surg (Abingdon). 2024 Dec;29(1):2345066. doi: 10.1080/24699322.2024.2345066. Epub 2024 Jun 11. Comput Assist Surg (Abingdon). 2024. PMID: 38860617
-
Development and validation of a machine learning-based risk prediction model for stroke-associated pneumonia in older adult hemorrhagic stroke.Front Neurol. 2025 Jun 18;16:1591570. doi: 10.3389/fneur.2025.1591570. eCollection 2025. Front Neurol. 2025. PMID: 40606135 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
References
-
- Cuschieri A. Laparoscopic cholecystectomy. J R Coll Surg Edinb 1999;44:187-92. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
