Advances in the Epigenetic Mechanisms of Diabetic Nephropathy Pathogenesis
- PMID: 40755747
- PMCID: PMC12318523
- DOI: 10.2147/DMSO.S507171
Advances in the Epigenetic Mechanisms of Diabetic Nephropathy Pathogenesis
Abstract
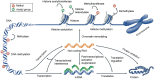
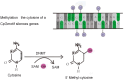
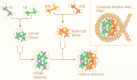
Diabetic nephropathy (DN) is one of the most severe microvascular complications of diabetes and a leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD). While traditional research has linked the onset of DN to factors such as metabolic dysregulation, inflammation, and oxidative stress, these mechanisms alone fail to fully explain the complex pathological features and individual variability of DN. In recent years, epigenetic research has provided new insights, revealing the critical roles of DNA methylation, histone modifications, and non-coding RNAs in the development of DN. DNA methylation regulates gene expression by altering methylation levels in promoter regions, affecting genes involved in inflammation and fibrosis. Histone modifications, including acetylation and methylation, influence gene transcription by altering chromatin structure. Additionally, non-coding RNAs, such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), play essential roles in gene expression networks. This review summarizes the latest advances in understanding these epigenetic mechanisms in DN pathogenesis, explores their roles in regulating inflammation, fibrosis, and cell damage, and discusses their potential applications in the diagnosis and treatment of DN. Further investigation into epigenetic modifications holds promise for identifying novel diagnostic markers and personalized therapeutic strategies for DN.
Keywords: DNA methylation; diabetic nephropathy; epigenetics; histone modifications; non-coding RNAs.
© 2025 Zhang et al.
Conflict of interest statement
The authors declare that they have no competing interests in this work.
Figures
Similar articles
-
A systematic review of epigenetic interplay in kidney diseases: Crosstalk between long noncoding RNAs and methylation, acetylation of chromatin and histone.Biomed Pharmacother. 2024 Jul;176:116922. doi: 10.1016/j.biopha.2024.116922. Epub 2024 Jun 12. Biomed Pharmacother. 2024. PMID: 38870627
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Regulation of pyroptosis in diabetic nephropathy by long non-coding and circular RNAs.Clin Exp Med. 2025 Jun 18;25(1):208. doi: 10.1007/s10238-025-01740-w. Clin Exp Med. 2025. PMID: 40531430 Free PMC article. Review.
-
Epigenetic alterations in prostate cancer: the role of chromatin remodeling.Epigenomics. 2025 Jul 22:1-25. doi: 10.1080/17501911.2025.2535938. Online ahead of print. Epigenomics. 2025. PMID: 40694614 Review.
-
Research progress on non-coding RNA regulatory networks and targeted therapy in diabetic nephropathy.Front Endocrinol (Lausanne). 2025 Jul 29;16:1625307. doi: 10.3389/fendo.2025.1625307. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40801037 Free PMC article. Review.
References
-
- Tang G, Li S, Zhang C, Chen H, Wang N, Feng Y. Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management. Acta Pharm Sin B. 2021;11(9):2749–2767. PMID: 34589395; PMCID: PMC8463270. doi: 10.1016/j.apsb.2020.12.020 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources