Additions of trastuzumab to preoperative chemotherapy or chemoimmunotherapy for patients with potentially resectable stage III to IVB HER2-positive gastric cancer
- PMID: 40755752
- PMCID: PMC12313684
- DOI: 10.3389/fimmu.2025.1624943
Additions of trastuzumab to preoperative chemotherapy or chemoimmunotherapy for patients with potentially resectable stage III to IVB HER2-positive gastric cancer
Abstract
Background: Whether the addition of trastuzumab to chemo(immuno)therapy for the preoperative treatment of patients with potentially resectable HER2-positive gastric cancer has clinical benefits remains to be explored. This real-world observational study was designed to evaluate the efficacy and safety of trastuzumab plus chemo(immuno)therapy for neoadjuvant or conversion therapy in patients with potentially resectable HER2-positive gastric cancer.
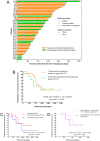
Methods: We retrospectively collected the clinical data of treatment-naïve patients with potentially resectable stage III to IVB HER2-positive gastric cancer who received preoperative therapy prior to D2 gastrectomy. The main outcomes of interest included tumour regression grade (TRG), treatment-related adverse events (TRAEs), and event-free survival (EFS).
Results: A total of 40 patients were included in the analysis, specifically, 27 patients (67.5%, 95% CI 0.520-0.799) received preoperative trastuzumab plus chemo(immuno)therapy, and 13 patients (32.5%, 95% CI 0.201-0.480) received chemo(immuno)therapy. All these patients subsequently underwent D2 gastrectomy. Regarding surgical outcomes, TRG0/1 rates were 33.3% (95% CI 0.186-0.522) in the trastuzumab-containing treatment group and 15.4% (95% CI 0.043-0.422) in the chemotherapy/chemoimmunotherapy group. Regarding safety, 66.7% (95% CI 0.478-0.814) of patients in the trastuzumab-containing treatment group and 61.5% (95% CI 0.355-0.823) of patients in the chemotherapy/chemoimmunotherapy group experienced preoperative TRAEs. The probabilities of EFS were not statistically significant between the two groups by the last follow-up.
Conclusion: Additions of trastuzumab to preoperative chemotherapy or chemoimmunotherapy resulted in a TRG0/1 rate of 33.3% among patients with potentially resectable HER2-positive gastric cancer, and the combined regimen exhibited a favourable safety profile.
Keywords: HER2-positive gastric cancer; immune checkpoint blockade; preoperative therapy; trastuzumab; tumour regression grade.
Copyright © 2025 Zhang, Tian, Wang, Song, Chen, Liu, Zhang, Huang, Jiang and Hou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Shitara K, Rha SY, Wyrwicz LS, Oshima T, Karaseva N, Osipov M, et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. (2023) 25:212–24. doi: 10.1016/S1470-2045(23)00541-7, PMID: - DOI - PubMed
-
- Janjigian YY, Al-Batran S-E, Wainberg ZA, Cutsem EV, Molena D, Muro K, et al. Pathological complete response (pCR) to 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) with or without durvalumab (D) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): Subgroup analysis by region from the phase 3, randomized, double-blind MATTERHORN study. J Clin Oncol. (2024) 42:LBA246–6. doi: 10.1200/JCO.2024.42.3_suppl.LBA246 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

