C-reactive protein induced T cell activation is an indirect monocyte-dependent mechanism involving the CD80/CD28 pathway
- PMID: 40755767
- PMCID: PMC12313514
- DOI: 10.3389/fimmu.2025.1622865
C-reactive protein induced T cell activation is an indirect monocyte-dependent mechanism involving the CD80/CD28 pathway
Abstract
Introduction: T cells are major components of the immune system. Their activation requires interaction between the T cell receptor and co-stimulatory molecules, crucial during infection, inflammation, and allogeneic rejection. Monomeric CRP (mCRP) is a known modulator of inflammation and particularly the innate immune response, however its interaction with T cells as part of the adaptive immune response remains unclear.
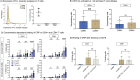
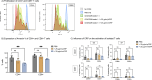
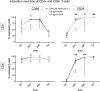
Methods: Peripheral blood mononuclear cells (PBMC) and T cells were isolated. Flow cytometric analysis was conducted to evaluate Fcγ receptor CD16 expression on T cells, the binding of CRP to T cells, and its impact on proliferation and apoptosis. T cell activation was assessed after 1, 2, 3, 5 and 7 days by assessing CD69 and CD25 expression, and under various conditions including coculture with monocytes and several inhibitory factors.
Results: T cells express CD16 that binds mCRP in a concentration-dependent manner, and particularly on activated T cells. While mCRP reduces apoptosis and accelerates proliferation in T cells, it does not independently activate them. However, activation of monocytes by mCRP leads to T cell activation, indicating a direct cell to cell interaction during CRP-induced activation. This effect could be alleviated by inhibition of the CD80/CD28 pathway.
Conclusion: CRP does not activate T Cells directly but via PI3-kinase-dependent activation of monocytes and subsequent CD80/CD28 cell to cell contact. The findings suggest the effects of CRP on T cells depend on their environment and the presence of other proinflammatory agents.
Keywords: Belatacept; C-reactive protein; CD80/CD28 pathway; T cells; innate immunity; monocytes.
Copyright © 2025 Thomé, Limmer, Brose, Zeller, Chevalier, Schäfer, Schneider, Lind, Christmann, Dreck, Kreuzaler, Braig, Peter, Pankratz and Eisenhardt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Interactions Between the Innate and Adaptive Immune Responses.Adv Exp Med Biol. 2025;1476:297-308. doi: 10.1007/978-3-031-85340-1_12. Adv Exp Med Biol. 2025. PMID: 40622548 Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Enhanced IL-15-mediated NK cell activation and proliferation by an ADAM17 function-blocking antibody involves CD16A, CD137, and accessory cells.J Immunother Cancer. 2024 Jul 24;12(7):e008959. doi: 10.1136/jitc-2024-008959. J Immunother Cancer. 2024. PMID: 39053944 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

