A multidimensional analysis of temporomandibular joint and ankle joint erosion in inflammatory arthritis
- PMID: 40755771
- PMCID: PMC12313558
- DOI: 10.3389/fimmu.2025.1560723
A multidimensional analysis of temporomandibular joint and ankle joint erosion in inflammatory arthritis
Abstract
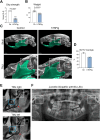
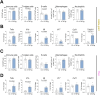
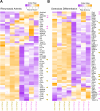
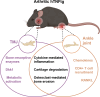
Rheumatoid arthritis (RA) and other inflammatory arthritis are systemic diseases that primarily affect the joints, characterized by synovial inflammation and progressive cartilage and bone degradation. The temporomandibular joint (TMJ) is reported to be involved in over 50% of RA cases, often leading to severe jaw pain and compromised oral function. Despite its prevalence, TMJ involvement is often underestimated, and its cellular and molecular mechanisms remain poorly understood. Due to the unique biological and functional properties of the TMJ, inflammatory pathways observed in other joints such as the well-studied ankle joint may not directly apply to the TMJ. This study aimed to establish a reliable inflammatory arthritis model for investigating TMJ-specific pathomechanisms. The human TNF-α transgenic (hTNFtg) mouse model effectively replicated TMJ pathology seen in arthritic patients, including increased synovial inflammation (p=0.0024) and severe bone loss (p=0.009) as compared to control mice assessed by micro-computed tomography and histomorphometry. These changes were driven by increased osteoclast numbers (p=0.0331) and upregulation of genes associated with bone resorption such as Acp5 (p=0.0003) and Ctsk (p=0.0025). Notably, we observed that the TMJ displays a unique pattern of immune cell infiltration and pro-inflammatory cytokine expression compared to the ankle joint, particularly with respect to T cell recruitment. These findings were further supported by bulk RNA sequencing, which revealed overall increased inflammation in both the ankle joint and TMJ of hTNFtg mice compared to the control group. Interestingly, while the expression of immune cell and pro-inflammatory cytokine-related gene sets was higher in the ankle joint, the TMJ showed increased expression of genes associated with energy consumption and bone resorption-related enzymes. These findings highlight the TMJ as a distinct anatomical site with heightened susceptibility to arthritis-related damage and emphasize the need for greater awareness and targeted research to improve disease management for affected individuals.
Keywords: bone erosion; human TNF-α transgenic mice; inflammatory arthritis; juvenile idiopathic arthritis temporomandibular joint; osteoclasts; rheumatoid arthritis; temporomandibular disorder.
Copyright © 2025 Andreev, Porschitz, Weidner, Song, Weider, Schett, Gölz and Bozec.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
DS-Modified Paeoniflorin pH-Responsive Lipid-Polymer Hybrid Nanoparticles for Targeted Macrophage Polarization in a Rat Model of Rheumatoid Arthritis.Int J Nanomedicine. 2025 Jul 12;20:8967-8992. doi: 10.2147/IJN.S516434. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40671689 Free PMC article.
-
Cytokines in saliva, serum, and temporomandibular joint synovial fluid in children with juvenile idiopathic arthritis: An explorative cross-sectional study.Pediatr Rheumatol Online J. 2025 Jun 17;23(1):66. doi: 10.1186/s12969-025-01118-y. Pediatr Rheumatol Online J. 2025. PMID: 40528176 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
-
- Zhang F, Wei K, Slowikowski K, Fonseka CY, Rao DA, Kelly S, et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol. (2019) 20:928–42. doi: 10.1038/s41590-019-0378-1, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

