CAR T-cells meet autoimmune neurological diseases: a new dawn for therapy
- PMID: 40755780
- PMCID: PMC12313632
- DOI: 10.3389/fimmu.2025.1604174
CAR T-cells meet autoimmune neurological diseases: a new dawn for therapy
Abstract
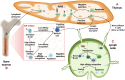
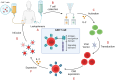
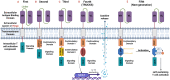
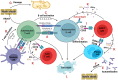
Autoimmunity and autoimmune diseases arise when the immune system erroneously targets self-antigens leading to tissue damage. Consequently, immunomodulatory and mainly immunosuppressive drugs comprise the conventional treatment in conditions such as systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis. However, many of these agents often fall short of providing a cure and have a limit on symptom management. This underscores the urgent need for even more advanced therapies for patients to constrain progressive disability. Therefore, currently, researchers explore the potential of chimeric antigen receptor (CAR) T-cell therapy for autoimmune diseases considering its success in cancer treatment and specifically in hematological malignancies. This review will examine recent advancements in CAR T-cell therapy for autoimmune disorders, highlighting how CAR T cells can be engineered to precisely target and eliminate autoreactive immune cells that drive these debilitating diseases, particularly those affecting the nervous system such as Multiple sclerosis, Myasthenia gravis, Neuromyelitis optica, Stiff-person syndrome, Autoimmune encephalitis, MOG-antibody disease and Chronic inflammatory demyelinating polyneuropathy. Also, through an analysis of preclinical and clinical data, we will assess the efficacy, safety, potential side effects and limitations of these innovative therapies. Lastly, we will underline the transformative potential of CAR T-cells in Autoimmune Neurology, offering a promising new hope for treatment where conventional therapies have failed.
Keywords: CAR T-cells; CRS; autoimmune neurological diseases; clinical trials; efficacy; limitations; preclinical data; safety.
Copyright © 2025 Chinas and Alexopoulos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

