PCK1 as a potential hub gene in distinguishing lactate metabolism between rheumatoid arthritis and osteoarthritis
- PMID: 40755786
- PMCID: PMC12318502
- DOI: 10.7717/peerj.19661
PCK1 as a potential hub gene in distinguishing lactate metabolism between rheumatoid arthritis and osteoarthritis
Abstract
Background: Lactate is notably involved in the advancement of rheumatoid arthritis (RA) and osteoarthritis (OA). Nevertheless, the causal association between these conditions and lactate remains uncertain. This study aims to use Mendelian randomization (MR) to investigate their relationship with lactate and understand the genetic differences in lactate metabolism between them.
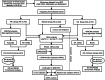
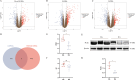
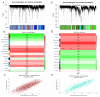
Methods: Genetic data for RA, OA, and lactate metabolism were obtained from GWAS, GEO, and MSigDB databases. MR analysis was performed using the inverse variance weighted (IVW) method. Differential gene expression analysis was conducted using the "limma" package, and Gene Set Enrichment Analysis (GSEA) was performed with GSEA software. Immune cell infiltration was assessed using the CIBERSORT platform. Validation of differentially expressed genes was carried out via Western blotting. Additionally, weighted gene co-expression network analysis (WGCNA) was employed to identify hub genes, while GO and KEGG analyses were performed to compare mechanistic differences between RA and OA. In vitro experiments were conducted to assess the effects of PCK1 on lactate secretion and cellular functions in RA-FLS.
Results: MR analysis indicated a causal relationship between RA and OA with lactate levels. Differential gene expression analysis revealed that PCK1 is a key gene underlying the metabolic differences in lactate levels between RA and OA. In vitro experiments demonstrated that knocking down PCK1 in RA-FLS affected lactate secretion, inhibited cell migration, and promoted apoptosis, suggesting its critical role in lactate metabolism. Additionally, GSEA analysis showed significant enrichment of PCK1 in the citrate cycle and gluconeogenesis signaling pathways in RA.
Conclusion: This study provides genetic evidence supporting the causal relationship between RA, OA, and lactate levels. Additionally, PCK1 is identified as a pivotal target implicated in the metabolic disparities of lactate between RA and OA, highlighting its potential significance in RA therapeutics.
Keywords: Lactate metabolism; Osteoarthritis; PCK1; Rheumatoid arthritis.
©2025 Xin et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures






Similar articles
-
Genetic and molecular underpinnings of the link between rheumatoid arthritis and myasthenia gravis: Insights from GWAS and transcriptomic analyses.Clin Rheumatol. 2025 Sep;44(9):3399-3417. doi: 10.1007/s10067-025-07582-x. Epub 2025 Jul 19. Clin Rheumatol. 2025. PMID: 40681713
-
Identification and validation of CKAP2 as a novel biomarker in the development and progression of rheumatoid arthritis.Front Immunol. 2025 Jun 25;16:1606201. doi: 10.3389/fimmu.2025.1606201. eCollection 2025. Front Immunol. 2025. PMID: 40636121 Free PMC article.
-
Shared molecular biomarkers and therapeutic targets in rheumatoid arthritis and osteoarthritis: Focus on EIF3B, KHSRP, NCL, PDCD1LG2, and SLC25A37.Cytokine. 2025 Sep;193:156988. doi: 10.1016/j.cyto.2025.156988. Epub 2025 Jun 30. Cytokine. 2025. PMID: 40592131
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.Health Technol Assess. 2008 Apr;12(11):1-278, iii. doi: 10.3310/hta12110. Health Technol Assess. 2008. PMID: 18405470
-
Mobile bearing vs fixed bearing prostheses for posterior cruciate retaining total knee arthroplasty for postoperative functional status in patients with osteoarthritis and rheumatoid arthritis.Cochrane Database Syst Rev. 2015 Feb 4;2015(2):CD003130. doi: 10.1002/14651858.CD003130.pub3. Cochrane Database Syst Rev. 2015. PMID: 25650566 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

